Introduction
It began as a heart medicine, became a symbol of male vitality, and now, unexpectedly, may find itself on the front lines of neurodegenerative research. Sildenafil, better known by its trade name Viagra®, has spent the past two decades helping millions reclaim an aspect of life once lost to age or illness. Yet, beneath its familiar reputation as a phosphodiesterase-5 (PDE5) inhibitor for erectile dysfunction lies a molecular story that reaches far beyond vascular smooth muscle — into the realm of neurons, synapses, and cognition.
Alzheimer’s disease (AD), the most common cause of dementia, continues to defy modern medicine. With over 50 million individuals affected worldwide and a projected cost surpassing USD 1 trillion annually within the next decade, AD remains one of humanity’s greatest biomedical and socioeconomic challenges. Despite intensive efforts, disease-modifying therapies remain elusive. The past two decades have seen hundreds of failed clinical trials, countless hypotheses tested and discarded, and a growing awareness that the solution may not lie in novel molecules, but rather in repurposing familiar ones.
This is precisely where sildenafil enters the story. Beyond its well-known hemodynamic effects, sildenafil exerts subtle yet profound influence on the brain’s nitric oxide–cGMP signaling — a pathway intimately linked to synaptic plasticity and memory formation. Recent animal studies have demonstrated improved cognition and reduced amyloid-β burden with PDE5 inhibition. But can a vasodilator truly guard against dementia in humans?
A team of researchers from the National University of Singapore and Duke-NUS Medical School sought to answer this question in 2025, conducting the first systematic review and meta-analysis examining the association between sildenafil use and the risk of Alzheimer’s disease. The findings — a two-fold reduction in AD risk among sildenafil users — have reawakened interest in the drug’s neuroprotective potential and raised provocative questions about the future of dementia prevention.
The Biology Behind the Hypothesis: Why Sildenafil Might Matter to the Brain
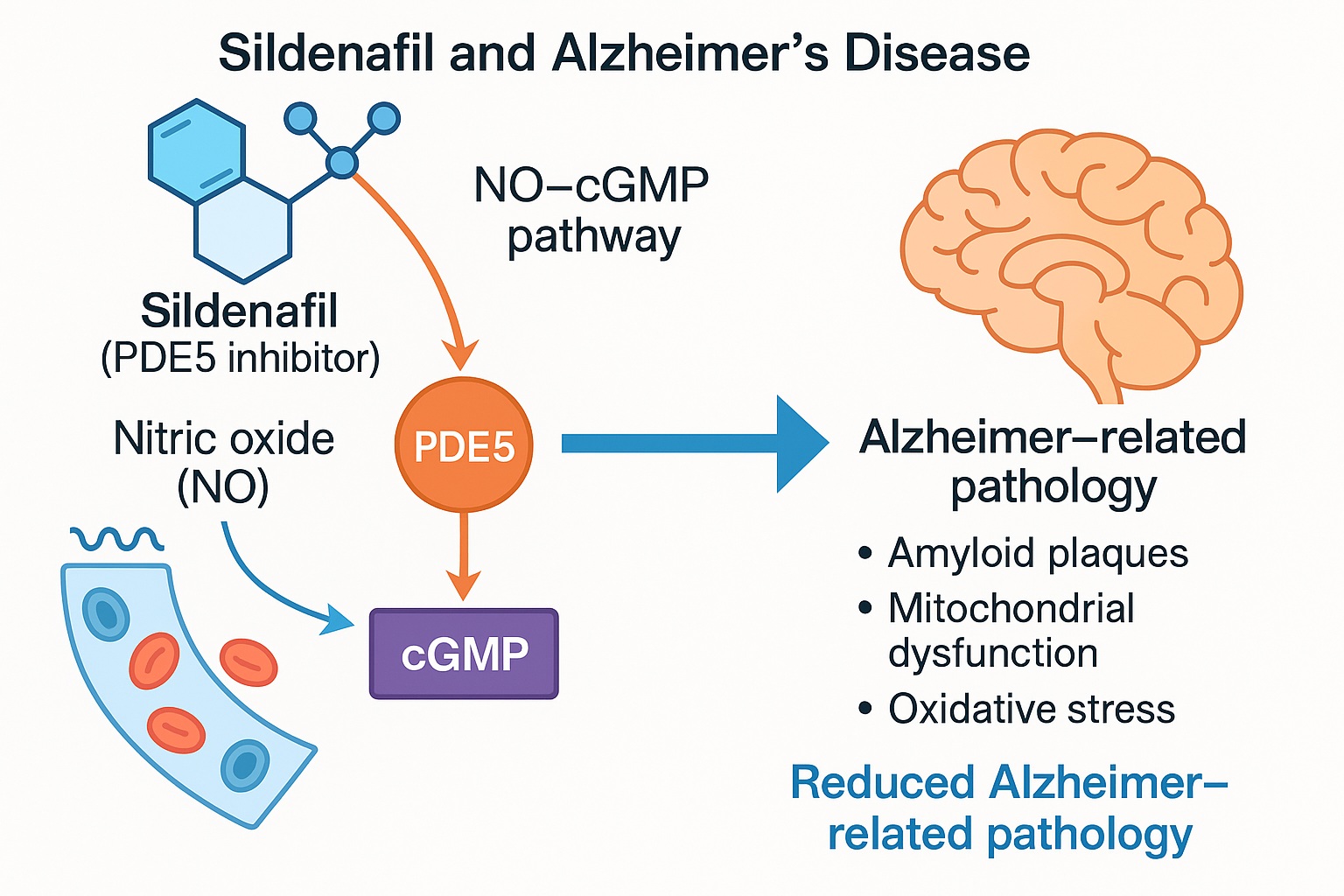
Sildenafil’s primary mechanism is familiar: inhibition of PDE5, an enzyme responsible for the degradation of cyclic guanosine monophosphate (cGMP). By preventing cGMP breakdown, sildenafil prolongs the signaling cascade initiated by nitric oxide (NO), leading to vasodilation and enhanced blood flow. This mechanism, however, extends well beyond penile tissue. PDE5 is expressed in cerebral vasculature, neurons, and glial cells, where NO-cGMP signaling regulates not only perfusion but also neuroplasticity, mitochondrial biogenesis, and oxidative balance.
In the Alzheimer’s brain, this signaling axis is disrupted. Amyloid-β peptides and tau pathology impair endothelial nitric oxide synthase (eNOS), leading to reduced NO production, diminished cGMP levels, and a cascade of vascular and metabolic dysfunctions. The result is impaired cerebral perfusion and neuronal energy failure — both key contributors to cognitive decline.
Preclinical models have consistently shown that restoring cGMP signaling via PDE5 inhibition can:
- Enhance synaptic plasticity through activation of cAMP response element-binding protein (CREB).
- Reduce amyloid-β deposition in the hippocampus.
- Promote mitochondrial biogenesis through activation of PGC-1α, a master regulator of cellular energy metabolism.
- Improve cerebral blood flow, mitigating ischemic hypoperfusion.
These mechanisms collectively provide a plausible biological foundation for sildenafil’s potential neuroprotective effect. What had remained uncertain until recently was whether such effects extend to humans at population scale.
The Study at a Glance: A Meta-Analysis of 885,380 Patients
The meta-analysis conducted by Chua et al. (2025) represents the most comprehensive evaluation to date of sildenafil’s relationship with Alzheimer’s risk. The researchers systematically searched MEDLINE and Embase from database inception to March 2024, identifying over 400 studies. After rigorous screening and quality assessment using the Newcastle-Ottawa Scale, five high-quality cohort and case-control studies were included, encompassing a total of 885,380 participants.
Study Designs and Data Sources
The included studies drew upon diverse real-world datasets — from U.S. Medicare and MarketScan claims to the UK IQVIA Medical Research Data and specialized registries such as CaPSURE. Collectively, these databases provided longitudinal information on drug exposure, comorbidities, and incident Alzheimer’s diagnoses. Participants in the treatment arms had received sildenafil or other PDE5 inhibitors (such as tadalafil or vardenafil), while control groups comprised matched non-users.
Analytical Approach
The investigators employed a random-effects meta-analysis to account for inter-study variability. Both hazard ratios (HR) and risk ratios (RR) were extracted and pooled using Review Manager 5.4.1 and RStudio 4.3.3, with sensitivity analyses and influence diagnostics (Baujat and leave-one-out plots) ensuring robustness.
The primary outcomes were twofold:
- Association between sildenafil use and risk of Alzheimer’s disease.
- Association between any PDE5 inhibitor use and AD risk.
A secondary subgroup analysis assessed gender-specific differences.
Key Findings: A Two-Fold Reduction in Alzheimer’s Risk
The pooled data yielded a compelling result. Individuals using sildenafil demonstrated a hazard ratio of 0.47 (95% CI: 0.27–0.82, p < 0.001) for developing Alzheimer’s disease compared with non-users — indicating a roughly 53% lower risk.
When all PDE5 inhibitors were considered together, the risk reduction remained significant (RR = 0.55, 95% CI: 0.38–0.80, p = 0.002). This association persisted even after excluding outlier studies and performing sensitivity analyses. Importantly, the protective effect was most consistent among male participants, likely reflecting the demographic profile of sildenafil users.
Interpreting the Numbers
To put these findings in perspective: across the included studies, more than 1770 patients in the treatment group and 3050 in the control group developed Alzheimer’s disease during follow-up. While the absolute numbers may appear small, the relative difference remained statistically and clinically meaningful. In large populations, even modest relative risk reductions translate into substantial public-health impact.
The Mechanistic Bridge: How a PDE5 Inhibitor Might Protect the Aging Brain
The apparent epidemiological link between sildenafil and reduced Alzheimer’s incidence invites mechanistic interpretation. Several interconnected biological processes are thought to underlie this effect.
1. Restoration of NO-cGMP Signaling
In Alzheimer’s pathology, reduced NO availability impairs cGMP-dependent signaling. Sildenafil restores this pathway by preventing cGMP degradation, enhancing protein kinase G (PKG) activation, and promoting downstream neuroprotective gene expression. This re-establishes synaptic efficiency and resilience.
2. Improved Cerebral Perfusion
Cerebrovascular dysfunction is a recognized hallmark of AD. Sildenafil’s vasodilatory action augments regional blood flow, particularly in the hippocampus — a region notoriously susceptible to hypoperfusion. Functional MRI studies in humans have confirmed that acute sildenafil administration increases hippocampal perfusion, theoretically supporting neuronal metabolism.
3. Mitochondrial Rescue and Oxidative Balance
By activating PGC-1α, sildenafil enhances mitochondrial biogenesis and the expression of antioxidant enzymes. This counteracts oxidative stress, one of the earliest contributors to neurodegeneration. Improved mitochondrial function translates to better synaptic energy supply and reduced vulnerability to amyloid-induced toxicity.
4. Amyloid Modulation
In animal models, sildenafil reduces amyloid-β accumulation in the hippocampus, possibly through enhanced clearance or decreased production. Though human confirmation remains pending, these data provide a biological rationale for disease modification rather than mere symptomatic relief.
Taken together, these mechanisms align with the central pathophysiological themes of Alzheimer’s disease — vascular insufficiency, mitochondrial dysfunction, oxidative stress, and synaptic loss. That a single molecule might beneficially influence all four is both remarkable and worthy of further exploration.
Reconciling Conflicting Evidence: Why Not All Studies Agree
Science rarely moves in straight lines. While the overall meta-analytic result favors sildenafil, individual studies have reported varying outcomes. Some, such as Fang et al. (2021) and Adesuyan et al. (2024), showed clear protective associations, whereas Desai et al. (2022) found no significant benefit.
Several factors may explain these discrepancies:
- Study Population Differences: Some cohorts included patients with pulmonary hypertension or prostate cancer, conditions with distinct comorbidity patterns and drug exposures.
- Duration of Follow-Up: Alzheimer’s disease develops slowly. Studies with short observation periods (e.g., 150 days in Desai et al.) likely underestimated true incidence.
- Gender Bias: Most included cohorts were predominantly male, reflecting prescription demographics. Since women have higher baseline AD risk, underrepresentation may distort results.
- Exposure Misclassification: Retrospective datasets rely on prescription records, which may not reflect actual medication adherence.
Despite these limitations, the aggregate data consistently trend toward neuroprotection. Even if confounding cannot be entirely excluded, the biological plausibility strengthens the case for causality.
Translational Implications: From Erectile Function to Cognitive Preservation
The implications of these findings stretch well beyond curiosity. If sildenafil indeed confers protection against Alzheimer’s pathology, it represents a paradigm of drug repurposing — leveraging a safe, inexpensive, and globally available medication for an entirely different indication.
Advantages of Repurposing Sildenafil
- Established Safety Profile: With over two decades of clinical use and millions of prescriptions, sildenafil’s side-effect spectrum is well characterized and generally manageable.
- Central Nervous System Penetration: The drug crosses the blood–brain barrier, achieving concentrations sufficient to inhibit cerebral PDE5.
- Cost-Effectiveness: Generic sildenafil is inexpensive compared with novel Alzheimer’s therapeutics.
- Mechanistic Convergence: The NO-cGMP pathway intersects with multiple neurodegenerative mechanisms, offering pleiotropic benefits.
However, enthusiasm must be tempered by scientific rigor. Observational correlations cannot substitute for randomized controlled trials (RCTs). To definitively establish causality, prospective RCTs with standardized neuroimaging, biomarker, and cognitive endpoints are essential.
The Gender Gap: Why Female Representation Matters
An intriguing aspect of the meta-analysis is its gender imbalance. Across the five included studies, females constituted less than 0.2% of participants in the pooled sildenafil analysis, with the notable exception of Desai et al., which included 69% women. This reflects prescribing patterns — sildenafil remains predominantly used by men — but it also limits generalizability.
Epidemiologically, women are twice as likely to develop Alzheimer’s disease as men, owing to a complex interplay of hormonal, genetic, and metabolic factors. Estrogen decline, mitochondrial vulnerability, and differences in APOE genotype distribution all contribute to heightened risk. Whether sildenafil’s molecular effects differ by sex remains unknown. Estrogen itself modulates NO-cGMP signaling, suggesting that therapeutic responses could vary substantially between genders.
Future studies must explicitly include female cohorts, perhaps exploring alternative PDE5 inhibitors such as tadalafil, which is increasingly prescribed for non-sexual indications (e.g., pulmonary hypertension) in both sexes. Without gender-balanced data, the field risks perpetuating an evidence gap that mirrors the very inequities medicine seeks to eliminate.
Limitations and the Path Ahead
The authors of the meta-analysis were appropriately cautious in interpreting their findings. They acknowledged several key limitations:
- Retrospective Study Designs: All included data were observational. This introduces potential confounding, selection bias, and incomplete control of comorbidities.
- Uncertain Dosage and Compliance: Claims databases record prescriptions, not actual ingestion or dose consistency.
- Unmeasured Covariates: Lifestyle factors, concurrent medications, and socioeconomic determinants were not uniformly available.
- Limited Female Data: As discussed, the male-dominant samples constrain extrapolation.
Despite these constraints, the meta-analysis provides the strongest human evidence yet for a protective association between sildenafil and Alzheimer’s disease. It sets the stage for future double-blind randomized trials, ideally incorporating neuroimaging biomarkers (e.g., amyloid PET, hippocampal perfusion MRI) and CSF or plasma Aβ/tau quantification to elucidate mechanism and magnitude.
Beyond Alzheimer’s: Wider Neurological Horizons for Sildenafil
The story need not end with Alzheimer’s disease. PDE5 inhibition influences a broad array of cerebrovascular and neurodegenerative processes. Experimental studies have demonstrated that sildenafil:
- Improves cognitive performance in rodent models of vascular dementia and ischemic stroke.
- Enhances neurogenesis in post-stroke recovery by stimulating neural progenitor cells.
- Reduces oxidative stress and neuronal apoptosis in aging models.
- Augments endothelial function, benefiting overall cerebrovascular health.
These pleiotropic effects hint at a broader therapeutic potential across the dementia spectrum — from vascular cognitive impairment to mixed pathology and even Parkinsonian disorders. While each condition presents unique challenges, the shared underpinning of vascular and metabolic dysfunction makes PDE5 inhibition an attractive avenue for exploration.
A Note of Scientific Irony
There is something delightfully ironic about a drug designed to restore vitality in one aspect of aging now being investigated to preserve vitality in another — the mind. Pharmacology has a long history of serendipitous discoveries: penicillin from mold, sildenafil from failed angina trials, and now possibly cognitive preservation from a pill once synonymous with romance. It is a reminder that biology rarely draws strict boundaries; vascular, neural, and metabolic health are threads of the same physiological fabric.
Conclusion
The 2025 meta-analysis by Chua et al. delivers a cautiously optimistic message: regular sildenafil use is associated with roughly half the risk of developing Alzheimer’s disease. The biological rationale — restoration of NO-cGMP signaling, improved cerebral perfusion, enhanced mitochondrial function, and amyloid modulation — is both coherent and compelling. Yet, as every good scientist knows, correlation is not causation.
Nevertheless, the findings open a new chapter in the quest to prevent Alzheimer’s disease. They underscore the potential of drug repurposing — mining the pharmacological past to illuminate the neurological future. With proper clinical trials, standardized dosing, and inclusion of diverse populations, sildenafil could one day transition from a therapy of intimacy to one of memory.
In the meantime, it stands as a fascinating case study in how molecular pharmacology, epidemiology, and a touch of scientific curiosity can converge to challenge our understanding of aging and cognition.
FAQ
1. Does taking sildenafil actually prevent Alzheimer’s disease?
Not conclusively — at least not yet. Current evidence shows a strong association between sildenafil use and lower Alzheimer’s incidence, but causality remains unproven. Controlled clinical trials are needed to confirm whether sildenafil actively prevents disease or simply correlates with healthier lifestyles or comorbidities.
2. How might sildenafil protect the brain?
Sildenafil enhances nitric oxide–cGMP signaling, improving cerebral blood flow, reducing oxidative stress, and supporting mitochondrial and synaptic function. These effects may mitigate key processes involved in Alzheimer’s pathology, including amyloid accumulation and vascular dysfunction.
3. Is it safe or advisable to use sildenafil for cognitive protection?
No clinical guidelines currently recommend sildenafil for this purpose. While the drug is generally safe for approved indications, off-label use without supervision can be risky, particularly in individuals with cardiac or vascular conditions. Its potential role in neuroprotection remains an exciting but still experimental frontier.