Introduction
Vision, a process so deceptively simple to the observer, is one of the most intricate feats of neurophysiology. It begins with the delicate photoreceptors of the retina — biological sensors that transform photons into neural signals — and culminates in the organized chaos of cortical interpretation. The fidelity of this process depends upon an orchestra of molecular and electrical events, among which cyclic guanosine monophosphate (cGMP) and phosphodiesterases (PDEs) play leading roles. When one interferes with these molecular players, the consequences ripple through the visual system like discordant notes in an otherwise harmonious score.
Sildenafil citrate, globally recognized as Viagra®, was developed as a selective inhibitor of phosphodiesterase type 5 (PDE5), primarily for its vasodilatory effects in erectile dysfunction. However, it exhibits a notable affinity for phosphodiesterase type 6 (PDE6), an enzyme expressed almost exclusively in the photoreceptor layer of the retina. This unintended target interaction provides an unexpected opportunity: sildenafil becomes not merely a pharmacological curiosity but a tool to probe the fragile balance of visual processing.
The study “Progressive Effects of Sildenafil on Visual Processing in Rats” offers one of the most comprehensive experimental evaluations of how sildenafil affects visual electrophysiology. By analyzing flash visual evoked potentials (VEPs) and steady-state visual evoked potentials (SSVEPs), the authors explored how acute and progressive PDE6 inhibition reshapes retinal signaling and cortical interpretation. The results reveal a fascinating narrative of transient disruption, adaptation, and recovery within the neural circuits of vision — one that illuminates the very foundations of phototransduction.
The Molecular Context: When cGMP Meets Its Match
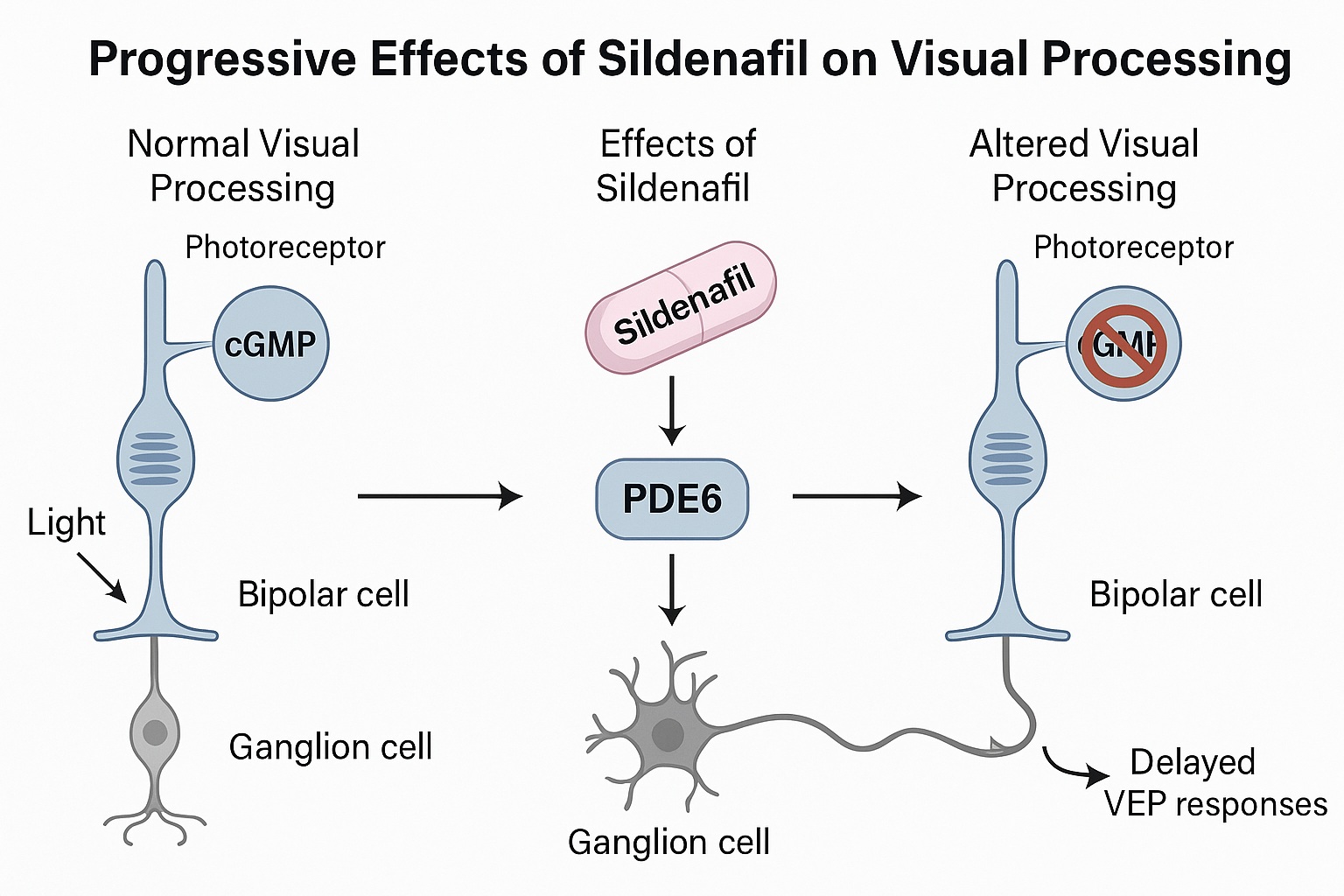
In the healthy retina, PDE6 serves as a gatekeeper for cGMP metabolism. Under the stimulus of light, rhodopsin activates a cascade that triggers PDE6 to hydrolyze cGMP, closing sodium channels in the outer segment of photoreceptors. The result is hyperpolarization — the first electrical step in seeing. When sildenafil inhibits PDE6, cGMP accumulates, preventing proper channel closure and interfering with the normal signal-to-noise ratio of phototransduction.
The effects of this interference are not limited to a single locus. Elevated cGMP in the photoreceptors disrupts ion flow, leading to altered membrane potentials that propagate through bipolar and ganglion cells. Even subtle shifts in cGMP dynamics can disturb temporal precision — a key determinant of contrast sensitivity and motion detection. Thus, what begins as a pharmacological side effect evolves into a mechanistic exploration of the retina’s dependence on enzymatic balance.
Interestingly, PDE6 inhibition is not uniformly detrimental. The study demonstrated that despite near-complete enzyme inhibition, visual processing was not abolished. This resilience suggests the presence of compensatory pathways — potentially involving intrinsically photosensitive retinal ganglion cells (ipRGCs) that respond via melanopsin-based, PDE-independent mechanisms. These cells, known for regulating circadian rhythms and pupillary reflexes, remind us that vision is not merely about image formation but also about synchronizing physiology with light itself.
Methods as Precision Instruments: A Modern Approach to Visual Electrophysiology
In the pursuit of understanding how sildenafil alters visual function, the study employed sophisticated electrophysiological assays in female Sprague-Dawley rats. Chronic electrode implantation in the visual cortex and superior colliculus allowed for simultaneous recording from two key nodes in the visual pathway: the cortical integrator and the midbrain relay. Such dual-site analysis provides an elegant window into how early retinal perturbations propagate through hierarchical processing layers.
The research design was deliberately progressive — spanning ten time points from 30 minutes to 28 hours after oral administration of sildenafil (50 mg/kg). This allowed for the tracking of temporal dynamics, from the acute pharmacological phase to recovery. The dose ensured near-complete PDE6 inhibition, based on known half-maximal inhibitory concentrations (IC50) in rod and cone cells. Pharmacokinetic profiling confirmed high free drug concentrations in both plasma and brain, reflecting systemic exposure sufficient to model human-like retinal effects.
Two complementary electrophysiological paradigms were used. Flash VEPs captured transient responses to discrete light stimuli, quantifying amplitude and latency — direct markers of neural transmission efficiency. SSVEPs, on the other hand, assessed sustained oscillatory responses to repetitive stimuli, offering insights into frequency-domain processing and harmonic generation. Together, they provided a multidimensional perspective on how sildenafil modulates both strength and timing in neural visual processing.
Sildenafil’s Electrophysiological Footprint: Reduced Amplitude, Delayed Timing
The most striking electrophysiological signature of sildenafil administration was a consistent reduction in the amplitude of VEPs across both the visual cortex and superior colliculus. This attenuation suggests weakened synaptic transmission or reduced responsiveness of retinal inputs. Simultaneously, increased latency — or delayed VEP peaks — indicated a slowing of neural conduction, possibly due to altered photoreceptor polarization or delayed neurotransmitter release.
These changes were most pronounced within the first five hours post-administration, correlating with peak plasma and brain concentrations of sildenafil. Interestingly, by the 28-hour mark, the visual system exhibited signs of recovery, reflecting the reversible nature of PDE6 inhibition. The superior colliculus, which receives direct retinal input, displayed more immediate and pronounced effects than the visual cortex — a logical outcome given its proximity to the initial site of perturbation.
The wavelength dependence of sildenafil’s effects adds a layer of intrigue. Blue and short-wave blue (SWB) stimuli produced distinct electrophysiological profiles compared to white light, highlighting the variable sensitivity of photoreceptor subtypes and ipRGC contributions. This spectral dependency suggests that sildenafil’s impact is not uniform across the visual spectrum, potentially explaining why patients often report transient blue-tinged vision.
The Superior Colliculus: A Retinal Mirror of Dysfunction
As the brain’s primary retinorecipient nucleus, the superior colliculus plays a pivotal role in orienting visual attention and reflexive gaze. In this study, its VEPs mirrored the retinal disturbances induced by sildenafil. Amplitude reductions and latency increases were again observed, particularly under white and SWB stimulation conditions. Notably, the early peaks of the waveform (P1, N1) — corresponding to initial synaptic transmission — were most affected, underscoring the compound’s impact on early visual relay circuits.
These findings reinforce the idea that sildenafil’s interference originates at the level of photoreceptor and bipolar cell signaling rather than higher cortical modulation. The superior colliculus’ sensitivity to blue light further aligns with the hypothesis that ipRGCs, which are maximally responsive around 480 nm, may provide partial compensation under these conditions. This interplay between photoreceptor and non-photoreceptor pathways illustrates the remarkable adaptability of the visual system under pharmacological stress.
Visual Cortex Adaptation: The Brain Compensates
While the superior colliculus showed pronounced deficits, the visual cortex exhibited a more nuanced response. Here, amplitude changes were significant but less uniform across wavelengths, suggesting that cortical integration mechanisms partially buffer peripheral disturbances. Neural circuits in the cortex, with their extensive lateral and feedback connections, likely compensate for upstream signal degradation through adaptive gain control and temporal filtering.
Nonetheless, sildenafil’s effects on latency persisted across multiple peaks, revealing that cortical neurons still process delayed inputs even when amplitude reductions subside. The persistence of temporal distortions underscores the cortex’s reliance on precise timing cues from the retina — an aspect that may explain the subjective “flickering” or “slow-motion” perception reported by human users of PDE5 inhibitors.
In translational terms, these cortical findings imply that electrophysiological monitoring — particularly VEP latency — could serve as a sensitive biomarker for drug-induced visual side effects. The rodent model thus offers a scalable paradigm for human risk assessment and therapeutic optimization.
Steady-State Visual Potentials: Rhythm Disrupted, Harmony Restored
The SSVEP results provide a complementary dimension to the story. Sildenafil significantly reduced the signal-to-noise ratio (SNR) of the steady-state response, particularly for the first harmonic under blue and SWB conditions. This reduction signifies diminished coherence between stimulus frequency and neural response — in essence, a “desynchronization” of the visual rhythm.
Curiously, the second harmonic, associated with more complex neural processing, was less affected or even transiently enhanced in some conditions. This dichotomy may reflect compensatory activity in ipRGC-mediated circuits, which are less dependent on PDE6. After approximately 28 hours, both harmonics normalized, consistent with drug clearance and restored retinal signaling fidelity.
From a systems neuroscience perspective, the recovery pattern underscores the retina’s plasticity and capacity for rapid biochemical re-equilibration. The transient nature of these effects parallels clinical observations: sildenafil-induced visual disturbances in humans are short-lived, reversible, and wavelength-specific.
Pharmacokinetics and Biochemical Implications
The pharmacokinetic data reveal that sildenafil reaches peak unbound brain concentrations (~800 nM) within 15 minutes, remaining above the IC50 for PDE6 for at least five hours. This corresponds to greater than 90% inhibition of retinal PDE6 during the critical observation window. The estimated half-life in rats (~3 hours) was longer than previously reported, likely due to dose-dependent metabolic saturation or slower absorption at higher concentrations.
This pharmacological profile aligns closely with human physiology, validating the rat as a translational model. Importantly, the reversible visual effects mirror clinical reports in which patients experience transient blue vision or mild visual field distortion, especially at high doses or when co-administered with other vasodilators. These parallels emphasize the model’s predictive value for assessing ocular side effects of PDE inhibitors.
Translational Relevance: Beyond Viagra’s Reputation
While sildenafil’s fame lies in its cardiovascular and sexual health applications, its value as a neuropharmacological probe is underappreciated. By transiently perturbing PDE6 activity, researchers can simulate conditions akin to retinitis pigmentosa or achromatopsia, diseases characterized by defective phototransduction. The study thus bridges pharmacology and neurodegeneration, demonstrating how a well-known drug can illuminate pathways of retinal disease.
Moreover, the dual-site electrophysiology approach offers a robust translational tool. Both VEP and SSVEP paradigms are applicable in humans, allowing direct comparison of drug-induced visual alterations across species. This strengthens the bridge between preclinical experimentation and clinical safety evaluation — a model of translational neuroscience at its best.
The Broader Picture: PDE Inhibition and Visual Neurophysiology
The retina, often described as an “outpost of the brain,” is exquisitely sensitive to metabolic and vascular fluctuations. Sildenafil’s dual action — elevating cGMP and modulating ocular blood flow — underscores the interconnectedness of biochemical and hemodynamic factors in vision. While PDE6 inhibition explains most electrophysiological disturbances, secondary effects such as transient intraocular pressure changes may also contribute.
Equally intriguing is the resilience of visual processing despite near-complete PDE6 blockade. This resilience highlights redundant signaling architectures — evolutionary safeguards that maintain visual perception even under pharmacological duress. Future studies might explore how chronic PDE modulation influences retinal plasticity, perhaps revealing new therapeutic targets for degenerative diseases.
Conclusion: Lessons from a Visual Experiment in Blue
The study’s findings elegantly confirm that visual processing is both robust and vulnerable — robust in its ability to recover from transient molecular disruption, yet vulnerable to even minor perturbations of its enzymatic balance. Sildenafil, in this context, becomes less a pharmacological artifact and more a diagnostic tool — a means to unravel the complex interplay between biochemical inhibition, electrophysiological timing, and perceptual integrity.
Ultimately, the research exemplifies the power of neuropharmacology as a window into sensory physiology. By observing how a well-characterized drug alters the neural symphony of vision, we gain deeper appreciation not only for the molecular foundations of sight but also for the delicate harmony that keeps our perception aligned with the world.
FAQ
1. Why does sildenafil affect vision in both humans and animals?
Sildenafil inhibits PDE6, an enzyme crucial for regulating cGMP in photoreceptors. This leads to transient alterations in retinal signaling, particularly affecting color discrimination and light sensitivity. The mechanism is conserved across species, explaining similar visual effects in humans and rats.
2. Are the visual side effects of sildenafil permanent?
No. The study confirms that sildenafil-induced visual disturbances are transient and reversible. Within approximately 24–28 hours, visual electrophysiological parameters in rats return to baseline, corresponding to drug clearance and restoration of normal PDE6 activity.
3. Can sildenafil serve as a research tool in visual neuroscience?
Absolutely. By selectively and reversibly inhibiting PDE6, sildenafil provides a unique pharmacological means to study phototransduction dynamics, visual evoked potentials, and retinal resilience. It serves as a valuable model for exploring both normal and pathological visual processing.