Introduction
Few natural compounds bridge the gap between ancient herbal lore and cutting-edge neuropharmacology as elegantly as icariin — the bioactive flavonol glycoside extracted from Epimedium species, better known by its evocative nickname, “horny goat weed.”
Once a fixture of Traditional Chinese Medicine (TCM) for restoring male vitality, icariin has recently entered the focus of modern biomedical research for a more scientifically defined reason: its phosphodiesterase-5 (PDE5) inhibitory activity, neurotrophic potential, and impact on endothelial and neuronal nitric oxide synthase (eNOS and nNOS).
The study led by Alan Shindel and colleagues at the University of California, San Francisco, presents a rigorous experimental investigation into icariin’s erectogenic and neurotrophic mechanisms, combining in vivo rat models of cavernous nerve injury with in vitro neuronal culture systems.
Their findings are remarkable not just for validating centuries-old traditional use, but for revealing how this natural compound may hold therapeutic promise for erectile dysfunction (ED) secondary to nerve injury — one of the most challenging conditions in sexual medicine.
A Natural PDE5 Inhibitor: Mechanistic Foundations
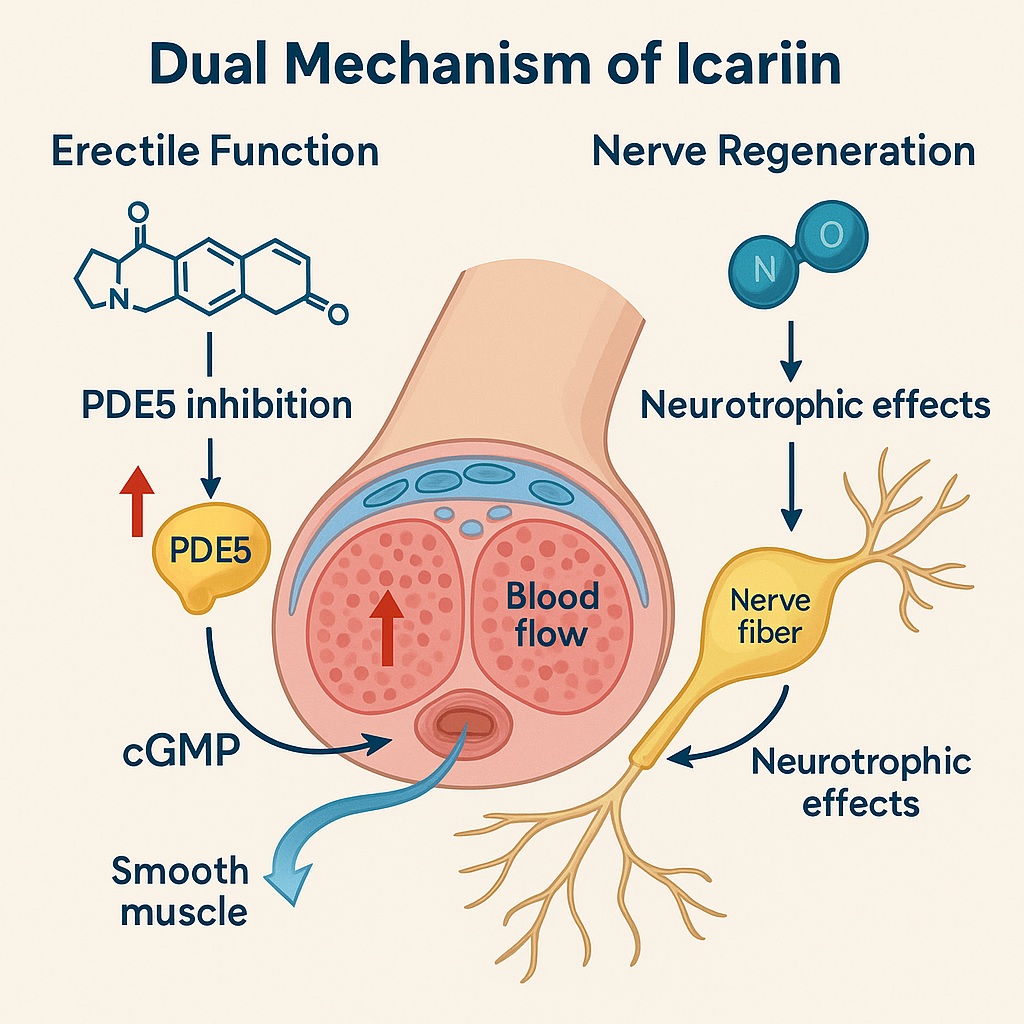
To appreciate icariin’s biomedical importance, one must first understand its pharmacological resemblance to sildenafil, the archetypal PDE5 inhibitor. PDE5, an enzyme expressed in corpus cavernosum smooth muscle, catalyzes the degradation of cyclic guanosine monophosphate (cGMP), the key messenger of nitric oxide–mediated vasodilation.
By blocking PDE5, icariin sustains cGMP levels, enhancing smooth muscle relaxation and blood inflow — the molecular essence of penile erection.
While native icariin’s PDE5 inhibition is relatively weak, chemical modification by addition of hydroxyethyl ether moieties enhances potency nearly 80-fold, approaching that of sildenafil.
Yet unlike purely synthetic analogs, icariin’s pleiotropic properties extend far beyond PDE5 blockade. It acts on endothelial function, oxidative stress, and apoptosis, and even demonstrates testosterone-mimetic and neuroregenerative activities.
At the molecular level, icariin enhances nitric oxide (NO) bioavailability, upregulates endothelial nitric oxide synthase (eNOS) expression, and attenuates caspase-3–mediated apoptosis.
These actions are complemented by its ability to increase cyclic nucleotide signaling and smooth muscle protein synthesis, thereby improving not only hemodynamics but also the structural integrity of erectile tissue.
The Experimental Model: From Crush to Cure
To simulate the neurogenic erectile dysfunction that commonly follows prostate surgery, researchers employed a cavernous nerve crush injury model in male Sprague-Dawley rats.
Following injury, animals were treated with daily oral icariin at doses of 1, 5, or 10 mg/kg for four weeks.
A separate group received a single pretest dose to distinguish acute PDE5-mediated effects from chronic neurorestorative outcomes.
Functional recovery was assessed through intracavernous pressure (ICP) responses during electrical stimulation of the cavernous nerves, normalized to mean arterial pressure (MAP).
Complementary biochemical analyses — including Western blotting and immunohistochemistry — quantified expression of nNOS, eNOS, calponin, and markers of apoptosis.
Finally, major pelvic ganglia (MPG) were cultured in vitro to assess neurite outgrowth under exposure to icariin, sildenafil, or control media.
This comprehensive design elegantly bridged vascular physiology, molecular biology, and neurobiology, providing an integrative perspective rarely seen in natural-product pharmacology.
Results: Restoring Function Through Dual Mechanisms
Enhanced Penile Hemodynamics
Rats treated with low-dose icariin (1 mg/kg) showed significant improvements in ICP/MAP ratios compared with untreated controls.
Interestingly, the lowest dose outperformed higher doses, suggesting a non-linear dose–response curve — possibly due to receptor saturation or biphasic pharmacodynamics.
These findings indicate that icariin restored erectile function not through a single acute vasodilatory event, but likely via chronic neurovascular repair.
Animals receiving only a single dose before testing did not exhibit measurable benefit, reinforcing the concept that sustained exposure, rather than immediate PDE5 inhibition, is critical for recovery.
Molecular Regeneration: Nitric Oxide Synthase Upregulation
Icariin-treated animals demonstrated marked upregulation of nNOS and calponin, both of which are vital to neuronal integrity and smooth muscle contractility.
nNOS, localized to nitrergic neurons of the cavernous nerves, is the main enzymatic source of nitric oxide that initiates penile erection.
Calponin, a contractile protein, reflects preservation of corporal smooth muscle architecture, a key determinant of penile rigidity.
Although eNOS expression trends were variable, histological staining suggested enhanced endothelial nitric oxide production, further contributing to improved perfusion and vascular health.
Neurotrophic Action in Culture
Perhaps the most intriguing finding emerged from the MPG culture assay.
When exposed to icariin, pelvic ganglion neurons exhibited significantly greater neurite outgrowth compared to both control and sildenafil-treated specimens.
This observation — that icariin stimulates neuronal regeneration whereas sildenafil does not — distinguishes the compound as not merely a PDE5 inhibitor, but a neurotrophic agent with potential applications in peripheral nerve injury recovery.
Hormonal Effects: A Complex Relationship
Serum testosterone levels displayed a paradoxical, dose-dependent pattern.
Low-dose icariin slightly elevated testosterone, consistent with previous reports of androgenic activity, whereas higher doses (≥10 mg/kg) suppressed serum levels.
Luteinizing hormone (LH) remained unchanged across groups, suggesting that icariin’s hormonal modulation acts peripherally rather than through the hypothalamic–pituitary axis.
This nuanced endocrine response underscores that more is not always better — a principle equally true in herbal pharmacology and clinical pharmacodynamics.
Mechanistic Insights: The Neurovascular Symphony of Icariin
The convergence of hemodynamic, molecular, and neurotrophic evidence suggests a multifactorial mechanism by which icariin restores erectile function.
Its effects can be broadly categorized into vascular modulation, neuronal regeneration, and anti-apoptotic cytoprotection.
1. Vascular Modulation via NO–cGMP Pathway
Icariin enhances NO-cGMP signaling by inhibiting PDE5 and upregulating eNOS.
Increased cGMP levels trigger smooth muscle relaxation through protein kinase G activation, leading to reduced intracellular calcium and subsequent vasodilation.
This re-establishes corporal compliance and endothelial homeostasis, reversing the microvascular dysfunction that follows nerve injury.
2. Neuronal Regeneration Through nNOS and Growth Signaling
Upregulation of nNOS not only improves erectile physiology but also reflects axonal survival and neurite extension.
In vitro, icariin activates pathways akin to nerve growth factor (NGF) and vascular endothelial growth factor (VEGF) signaling, promoting neurite elongation and synaptic stabilization.
These findings suggest activation of PI3K–Akt–CREB cascades, central to neuronal survival and plasticity.
3. Anti-Apoptotic and Antioxidant Actions
Icariin’s ability to suppress caspase-3 expression and oxidative damage, previously demonstrated in endothelial models, may also protect neurons and smooth muscle cells from degenerative loss after injury.
By attenuating apoptosis and reactive oxygen species, it fosters a microenvironment conducive to regeneration rather than scarring.
Collectively, these actions position icariin as a neurovascular modulator — one capable of addressing both the neural and vascular components of erectile dysfunction, a duality rarely achieved by synthetic PDE5 inhibitors alone.
Comparative Context: Icariin vs. Sildenafil and Other PDE5 Inhibitors
While sildenafil remains the gold standard in pharmacological ED management, its mechanism is purely vasodilatory and temporally limited.
Icariin, by contrast, exhibits long-term restorative effects, potentially improving underlying tissue and nerve integrity.
In the MPG culture study, sildenafil failed to enhance neurite outgrowth, underscoring the distinction between transient functional enhancement and true biological repair.
Moreover, icariin’s broader pharmacodynamic spectrum includes mitochondrial protection, angiogenesis promotion, and anti-inflammatory modulation, which collectively contribute to sustained tissue recovery.
From a translational perspective, this suggests that icariin could serve as an adjunct or successor to PDE5 inhibitors in cases of post-surgical or neurogenic erectile dysfunction, where neural recovery is as crucial as vascular function.
Dose Matters: The Paradox of Potency
The counterintuitive observation that low-dose icariin (1 mg/kg) yielded superior results compared to higher doses raises important pharmacological questions.
Possible explanations include:
- Receptor desensitization: Excessive PDE5 inhibition or feedback regulation may dampen downstream signaling.
- Endocrine counterbalance: High doses may interfere with androgen metabolism or exert suppressive effects on steroidogenesis.
- Pharmacokinetic saturation: Icariin’s short half-life (~1 hour) may limit bioavailability, leading to nonlinear dose–response curves.
These findings highlight the delicate equilibrium between therapeutic efficacy and biological adaptation, reminding clinicians and researchers alike that “natural” does not equate to “risk-free.”
Translational Significance: From Rat to Human Rehabilitation
In the clinical world, post-prostatectomy erectile dysfunction remains a daunting challenge.
Even with nerve-sparing techniques, temporary or permanent cavernous nerve injury often leads to denervation atrophy, smooth muscle apoptosis, and fibrosis — changes that blunt responsiveness to PDE5 inhibitors.
If icariin’s neurotrophic and erectogenic effects translate to humans, it could redefine penile rehabilitation protocols by offering both functional restoration and neural recovery.
Its dual mechanism might also benefit conditions such as diabetic neuropathy, pelvic nerve trauma, or ischemic penile disorders.
Furthermore, as a naturally derived compound with an established safety profile in traditional medicine, icariin could serve as a foundation for semi-synthetic derivatives optimized for potency, bioavailability, and selectivity — a pharmacognostic bridge between herbal wisdom and molecular design.
Caveats and Future Directions
While Shindel et al.’s findings are robust and biologically plausible, several caveats must be acknowledged:
- The animal model may not fully replicate human nerve injury dynamics or chronic ED pathology.
- The sample size limits statistical power for higher-dose effects and hormonal variability.
- The short observation window (4 weeks) may underestimate long-term regenerative outcomes.
- Human pharmacokinetics of purified icariin remain poorly defined, necessitating dose-finding and safety trials.
Future research should pursue:
- Randomized controlled studies in larger animal models or early-phase human subjects.
- Mechanistic mapping of icariin’s interaction with neurotrophic signaling (e.g., BDNF, NGF, and SIRT1 pathways).
- Formulation innovations (e.g., nanoemulsions or sustained-release preparations) to overcome poor oral bioavailability.
- Comparative genomics and transcriptomics to identify molecular targets unique to icariin.
Only through such rigorous translational work can icariin transition from herbal curiosity to clinically validated neurovascular therapy.
Conclusion
Icariin stands as a compelling example of how traditional medicine and modern science can converge on shared biochemical truths.
By combining PDE5 inhibition, nitric oxide enhancement, and neurotrophic stimulation, this natural compound addresses both the vascular and neural dimensions of erectile dysfunction — an achievement few synthetic drugs can claim.
In the hands of researchers like Shindel and colleagues, horny goat weed has evolved from folklore to evidence-based pharmacology.
If future clinical trials confirm these findings, icariin may emerge not only as a restorative agent for sexual health but also as a model compound for nerve regeneration therapy.
As history often proves, nature has a way of whispering solutions long before science learns to listen. Icariin may well be one of those whispers — now amplified by molecular evidence.
FAQ
1. What makes icariin different from sildenafil or other PDE5 inhibitors?
Unlike conventional PDE5 inhibitors, icariin exhibits both erectogenic and neurotrophic effects. It not only enhances penile blood flow but also promotes nerve regeneration and smooth muscle preservation, offering potential long-term recovery benefits after nerve injury.
2. Can icariin increase testosterone levels?
Evidence is mixed. Low doses appear to mildly elevate testosterone, while higher doses may suppress it. These effects seem independent of luteinizing hormone, suggesting modulation of local enzymatic pathways rather than central endocrine control.
3. Is icariin safe and effective for human use?
Icariin has an excellent safety record in traditional herbal medicine, but its clinical efficacy and pharmacokinetics in humans remain under investigation. Purified, standardized preparations and controlled dosing are essential before therapeutic recommendations can be made.