Introduction
In the world of pharmacognosy, few natural sources remain as paradoxical as the Osage orange (Maclura pomifera)—a hardy North American tree with unremarkable fruit and yet remarkable chemistry.
While its bumpy, lime-green fruits have long been dismissed as mere curiosities of the prairie, recent pharmacological research has uncovered a trove of bioactive prenylated isoflavonoids that could reposition this plant from botanical oddity to pharmacological goldmine.
The recent study on Maclura pomifera extracts provides a comprehensive biochemical and pharmacodynamic analysis, elucidating their antioxidant, anti-inflammatory, antitumor, and antimicrobial activities. Beyond these, the study delves into the mechanistic depth of prenylated isoflavones, molecules whose structural diversity grants them exceptional biological versatility.
This article synthesizes the scientific essence of that study and explores how Osage orange–derived compounds might redefine therapeutic innovation in oxidative stress, cancer biology, and metabolic disorders.
The Chemistry of Power: Prenylated Isoflavonoids and Their Molecular Distinction
Isoflavonoids represent a class of phenolic secondary metabolites predominantly associated with the Fabaceae family. Their pharmacological activities are well documented in compounds such as genistein and daidzein, the hallmark flavonoids of soy.
What sets the Osage orange apart is the prenylation—the attachment of lipophilic isoprenoid groups to the flavonoid backbone. This seemingly minor structural modification dramatically alters cellular permeability, metabolic stability, and receptor binding affinity.
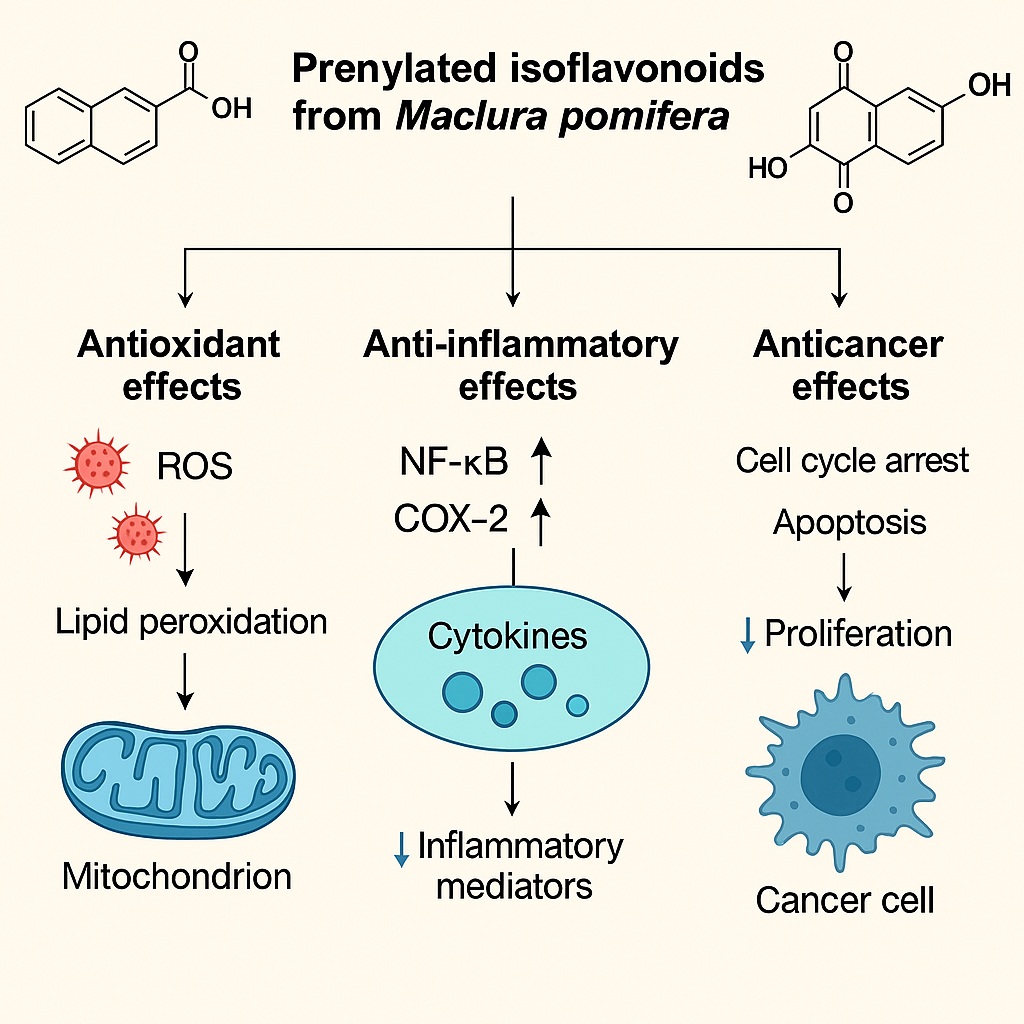
Among the key molecules identified in Maclura pomifera are osajin, pomiferin, and auriculasin, each a prenylated derivative with unique substituents conferring biological selectivity.
Prenylation enhances their interaction with lipid membranes and enables stronger inhibition of redox-sensitive enzymes such as NADPH oxidase and COX-2, thereby amplifying both antioxidant and anti-inflammatory effects.
Additionally, these molecules exhibit selective cytotoxicity against tumor cells—a property linked to their preferential accumulation in mitochondria and disruption of reactive oxygen species (ROS) homeostasis.
Unlike classical flavonoids, which often suffer from poor bioavailability, prenylated isoflavones display improved pharmacokinetics, rendering them potent candidates for systemic therapeutic applications.
Antioxidant Defense: Beyond Radical Scavenging
The antioxidant potential of Osage orange extracts extends beyond traditional free radical scavenging.
In biochemical assays, Maclura pomifera fruit extracts demonstrated strong ferric-reducing antioxidant power (FRAP) and DPPH radical inhibition, comparable or superior to standard antioxidants such as ascorbic acid.
Mechanistically, this effect arises from hydrogen-donating hydroxyl groups on the isoflavone ring, synergistically reinforced by the lipophilic prenyl chain, which anchors the molecule within the phospholipid bilayer.
This localization not only stabilizes cellular membranes under oxidative stress but also modulates intracellular redox signaling.
More importantly, prenylated isoflavonoids modulate endogenous antioxidant enzymes. Experimental data indicate increased expression of superoxide dismutase (SOD) and glutathione peroxidase (GPx), accompanied by a reduction in malondialdehyde (MDA), a biomarker of lipid peroxidation.
Thus, their antioxidant defense functions are enzyme-regenerative rather than merely chemical, positioning these compounds as cellular protectants rather than passive scavengers.
Anti-Inflammatory Activity: Modulating the COX–NF-κB Axis
Inflammation remains a common denominator in the pathophysiology of numerous chronic diseases. Maclura pomifera extracts exhibit potent anti-inflammatory properties, which the study attributes to inhibition of cyclooxygenase (COX) enzymes and suppression of nuclear factor-kappa B (NF-κB) signaling.
Prenylated isoflavonoids like pomiferin act as competitive inhibitors of COX-2, attenuating prostaglandin synthesis and downstream inflammatory cascades.
Simultaneously, their phenolic cores suppress the phosphorylation of IκBα, preventing nuclear translocation of NF-κB and thereby reducing transcription of pro-inflammatory mediators such as TNF-α, IL-6, and iNOS.
In macrophage cultures stimulated by lipopolysaccharide (LPS), treatment with Osage orange extract led to significant decreases in nitric oxide and cytokine release, without compromising cell viability.
This balance between efficacy and cytocompatibility is particularly valuable for developing safe, long-term anti-inflammatory therapies, unlike many synthetic NSAIDs that carry gastrointestinal or renal liabilities.
Anticancer Potential: Targeting Cellular Survival Pathways
The anticancer properties of Maclura pomifera extracts stand among its most promising pharmacological dimensions.
Multiple in vitro studies have revealed dose-dependent cytotoxicity of osajin and pomiferin against human cancer cell lines, including HeLa (cervical), MCF-7 (breast), and HepG2 (hepatocellular) models.
Mechanistically, these compounds exert their effects through ROS-mediated mitochondrial disruption, cell cycle arrest, and apoptosis induction.
At moderate concentrations, osajin upregulates p53 and Bax, while downregulating anti-apoptotic Bcl-2 expression.
The resulting mitochondrial membrane depolarization triggers cytochrome c release, activating caspase-9 and caspase-3, hallmark executors of programmed cell death.
Interestingly, the prenyl substituents enhance mitochondrial localization, facilitating selective targeting of cancer cells that depend heavily on oxidative phosphorylation.
This preferential cytotoxicity spares normal fibroblasts and epithelial cells, highlighting a potential therapeutic window rare in plant-derived compounds.
Moreover, evidence suggests that Maclura pomifera constituents may inhibit angiogenesis and metastasis, via downregulation of VEGF and MMP-9 expression.
Collectively, these data indicate a multi-target anticancer profile, engaging oxidative, apoptotic, and anti-proliferative pathways concurrently.
Antimicrobial Properties: Nature’s Subtle Antibiotic
Beyond antioxidant and anticancer mechanisms, Osage orange extracts display broad-spectrum antimicrobial activity, particularly against Gram-positive bacteria such as Staphylococcus aureus and Bacillus subtilis.
The activity against Gram-negative strains like E. coli and Pseudomonas aeruginosa is moderate, likely reflecting differences in cell wall permeability.
The prenyl moiety, once again, plays a central role. Its lipophilicity facilitates membrane insertion, causing leakage of intracellular contents and disruption of metabolic integrity.
At sublethal concentrations, prenylated isoflavones inhibit biofilm formation, a critical factor in antimicrobial resistance.
This property offers potential for anti-biofilm coatings in medical devices and natural preservative systems in pharmaceuticals or food applications.
Given the global crisis of antibiotic resistance, the search for plant-derived antibacterials such as those from Maclura pomifera represents a rational and ecologically sustainable direction.
Metabolic and Neuroprotective Implications
Recent pharmacological screening has expanded the potential of Osage orange compounds into metabolic and neuroprotective medicine.
Pomiferin, in particular, exhibits α-glucosidase inhibitory activity, suggesting anti-diabetic potential through postprandial glucose regulation.
Simultaneously, it attenuates oxidative stress in neuronal models, implicating benefits in neurodegenerative conditions like Alzheimer’s and Parkinson’s diseases.
Mechanistically, prenylated isoflavonoids activate the Nrf2–HO-1 antioxidant response pathway, promoting transcription of cytoprotective genes.
This mechanism reduces oxidative neuronal apoptosis, counteracts amyloid-β toxicity, and may enhance synaptic resilience.
In parallel, osajin demonstrates acetylcholinesterase inhibition, which could support cholinergic neurotransmission in cognitive impairment.
The convergence of antioxidant, anti-inflammatory, and neurotransmission-modulating effects makes Maclura pomifera extracts multifunctional neuroprotective candidates — a rarity even among plant-derived compounds.
Mechanistic Integration: The Cellular Symphony of Osage Orange
The pharmacological efficacy of Maclura pomifera arises from an intricate interplay of molecular mechanisms:
- Redox Modulation: By suppressing ROS formation and activating SOD/GPx, prenylated isoflavones maintain mitochondrial integrity.
- Inflammatory Control: Inhibition of NF-κB and COX-2 signaling reduces cytokine storm and inflammatory damage.
- Apoptotic Regulation: Balance of pro- and anti-apoptotic proteins restores homeostasis in hyperproliferative cells.
- Signal Transduction: Activation of Nrf2 and downregulation of MAPK/ERK cascades contribute to both cytoprotection and anti-tumor effects.
These molecular harmonies collectively define Maclura pomifera not as a single-target agent but as a multi-modal pharmacological system, capable of fine-tuning physiological responses across organ systems.
Safety and Toxicological Considerations
Toxicological profiling in both in vitro and in vivo studies indicates low cytotoxicity toward non-malignant cells and minimal organ toxicity at therapeutic concentrations.
However, high doses may induce mild hepatic enzyme elevation, likely reflecting metabolic processing of lipophilic prenylated compounds.
Long-term safety data remain limited, underscoring the need for chronic toxicity and genotoxicity assessments before clinical translation.
Nevertheless, the broad therapeutic index and metabolic stability reported so far suggest a favorable safety margin compared with synthetic polyphenols or chemotherapeutics.
Future Perspectives: From Prairie Fruit to Precision Medicine
The rediscovery of Maclura pomifera as a pharmacological resource embodies a broader trend in integrative natural product research—leveraging ancient biodiversity with modern mechanistic insight.
Its prenylated isoflavonoids exemplify how subtle structural modifications can amplify bioactivity, bridging the gap between natural diversity and drug design precision.
Future research directions should include:
- Pharmacokinetic modeling to optimize oral bioavailability and metabolic half-life.
- Synergistic studies with conventional drugs, exploring combinatorial effects in oncology and inflammation.
- Formulation innovations, such as nanoparticle encapsulation, to enhance solubility and tissue targeting.
- Molecular docking and transcriptomics, to map receptor-level interactions and downstream gene expression networks.
The clinical promise of Osage orange may ultimately extend beyond its extracts — inspiring synthetic analogs of prenylated isoflavones as scaffolds for next-generation therapeutics.
Conclusion
The fruits of Maclura pomifera, long overlooked by both botanists and pharmacologists, are emerging as a biochemical treasure chest.
Through the unique architecture of prenylated isoflavonoids, this humble plant manifests a pharmacological portfolio spanning antioxidant defense, anti-inflammatory regulation, antitumor potential, antimicrobial activity, and neuroprotection.
Far from being an obscure folk remedy, Osage orange now represents a platform for drug discovery, linking ethnobotanical heritage with molecular medicine.
Its compounds remind us that pharmacological sophistication need not be engineered—it can be refined by evolution and rediscovered by science.
FAQ
1. What makes the prenylated isoflavonoids from Maclura pomifera pharmacologically special?
Their lipophilic prenyl groups improve cell permeability, metabolic stability, and target selectivity. These structural advantages enhance antioxidant, anti-inflammatory, and anticancer activities beyond what typical flavonoids achieve.
2. Are Osage orange extracts safe for therapeutic use?
Preclinical data suggest good safety at therapeutic doses, with low cytotoxicity in normal cells. However, comprehensive human toxicity studies are still needed before formal clinical applications.
3. Could Maclura pomifera compounds become approved drugs?
Yes, but only after rigorous pharmacokinetic, toxicological, and clinical validation. Their strong mechanistic foundations and bioactive diversity make them promising candidates for future drug development, especially in oncology and metabolic disease.