Introduction
Few drugs have reshaped both medical science and cultural imagination as profoundly as sildenafil citrate, better known under its trade name Viagra. Initially developed as a cardiovascular drug in the 1980s, it became the first oral therapy for erectile dysfunction, igniting not only a pharmaceutical revolution but also an enduring fascination among scientists. What is less widely known, however, is that sildenafil has also become a celebrity compound in the world of crystallography — a molecule as versatile in the lab as it is in life.
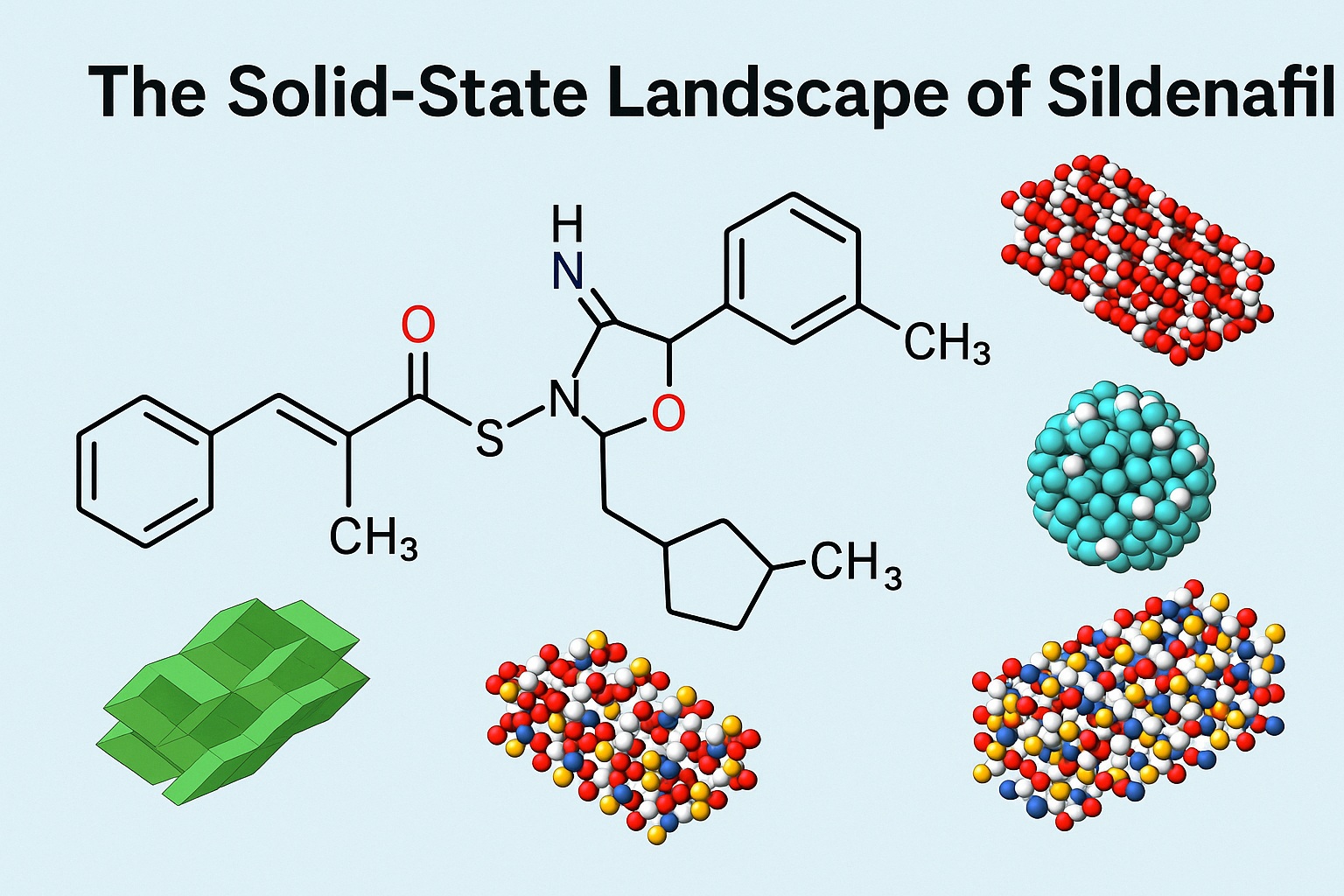
Behind the iconic blue tablet lies a world of complex solid-state forms — polymorphs, hydrates, solvates, salts, and cocrystals — that define the stability, solubility, and bioavailability of the active pharmaceutical ingredient (API). Over the decades, sildenafil has been the subject of intensive research by formulation scientists, crystallographers, and solid-state chemists. The molecule’s behavior in different solid environments has revealed an extraordinary structural flexibility, making it one of the most “promiscuous” crystal formers known in pharmaceutical science.
This article explores that fascinating world — the solid-state landscape of sildenafil — tracing its evolution from discovery to the present day. We will examine how structural diversity influences drug formulation, what drives the formation of different polymorphs, and why this knowledge remains central to both science and industry. By the end, you’ll see that Viagra’s success is not just a triumph of pharmacology, but also a quiet masterpiece of molecular architecture.
From Angina to Innovation: The Unexpected Journey of Sildenafil
In the late 1980s, Pfizer’s research division in Sandwich, England, embarked on a program to develop selective phosphodiesterase type 5 (PDE5) inhibitors for treating hypertension and angina pectoris. The logic was sound: PDE5 regulates cyclic guanosine monophosphate (cGMP), which controls smooth muscle tone and vasodilation. By inhibiting PDE5, blood vessels could remain dilated longer, easing cardiac workload.
The compound UK-92,480 — later christened sildenafil citrate — demonstrated strong PDE5 inhibition in vitro, but clinical trials were underwhelming in terms of cardiac effects. Yet participants consistently reported one peculiar “side effect”: frequent and firm erections. What began as a failed antihypertensive suddenly became a blockbuster therapy for erectile dysfunction (ED).
In March 1998, the U.S. FDA approved Viagra, marking the dawn of a new therapeutic era. By the end of that decade, Pfizer was selling more than 150 million tablets annually across 100 countries, earning over $1 billion a year. But while the public marveled at its social impact, scientists turned their gaze to the molecule’s structural secrets — and discovered that sildenafil’s complexity went far beyond the vascular system.
The Solid-State Universe: Polymorphism and Its Implications
To the layperson, a pill is a pill — a static object. To the solid-state chemist, however, every tablet is a crystalline universe, where molecules arrange themselves in intricate lattices governed by thermodynamics and kinetics. Sildenafil exemplifies this beautifully.
Approximately 90% of organic molecules are polymorphic, meaning they can crystallize in more than one distinct solid form. Each polymorph can exhibit dramatically different properties:
- Solubility determines how quickly the drug dissolves and is absorbed.
- Stability affects shelf life and resistance to environmental conditions.
- Bioavailability dictates therapeutic effectiveness.
- Patentability extends intellectual property lifespan and commercial protection.
In sildenafil’s case, over 42 distinct solid forms have been documented — including two anhydrous polymorphs, several solvates, amorphous forms, salts, and cocrystals. This remarkable diversity makes sildenafil not only a therapeutic agent but also a model compound for studying solid-state phenomena.
Understanding this diversity is more than academic curiosity. The appearance (or disappearance) of polymorphs can have catastrophic implications for pharmaceutical companies. A famous example outside sildenafil is the “disappearing polymorph” of ritonavir, which once halted production of an HIV drug. With sildenafil, researchers have meticulously mapped the landscape to ensure stability and predictability in its marketed form.
Anhydrous and Amorphous Forms: The Building Blocks of Stability
The sildenafil free base exists in two anhydrous crystalline polymorphs — Forms I and II — and one amorphous form. Form I is thermodynamically the most stable, typically crystallizing from most organic solvents. Form II, in contrast, is a metastable desolvate that appears after removal of solvent from specific solvated forms such as acetonitrile or propanenitrile.
At the molecular level, both polymorphs display a strong intramolecular hydrogen bond between the ethoxy and pyrimidine groups — a signature interaction that stabilizes their configuration. The difference lies in subtle conformational twists of the molecule, reflecting slight rearrangements of the same core geometry. Remarkably, these two forms are monotropically related — meaning Form I remains the more stable phase across all temperatures, while Form II converts to Form I upon heating or storage.
The amorphous form of sildenafil, produced by rapid cooling from its melt, presents an entirely different story. It lacks long-range crystalline order, yet exhibits high kinetic stability — remaining glassy and uncrystallized for extended periods at temperatures below 30°C. Above that, it tends to recrystallize swiftly. Such behavior makes the amorphous form attractive for short-term solubility enhancement but challenging for large-scale manufacturing due to its thermodynamic instability.
In practical terms, the anhydrous forms define stability and manufacturability, while the amorphous form offers solubility advantages at the expense of predictability. Together, they form the foundation of sildenafil’s solid-state portfolio.
Solvates and the Art of Molecular Companionship
Sildenafil’s free base, though hydrophobic, readily forms solvates — crystalline complexes incorporating solvent molecules into the lattice. No hydrates (water-based forms) of the free base have been reported, but multiple organic solvates exist, notably with acetonitrile, propanenitrile, toluene, anisole, and dioxane.
Two acetonitrile solvates (with 1:1 and 2:1 sildenafil-to-solvent ratios) are particularly significant. The 2:1 solvate acts as a precursor to the metastable anhydrous Form II upon desolvation. The propanenitrile solvate, conversely, transforms into the stable Form I when the solvent is removed.
The chemistry here borders on choreography: intermolecular hydrogen bonds and π–π stacking interactions dictate how solvent molecules nestle within the sildenafil lattice. Interestingly, certain aromatic solvates — such as those with anisole or toluene — disrupt the typical hydrogen bond motif by forming aromatic stacking arrangements instead.
What fascinates crystallographers is how these interactions mirror sildenafil’s own biological behavior. In the crystal structure of the human PDE5 enzyme bound to sildenafil, the same intramolecular hydrogen bond found in most solid forms is broken. A glutamine residue of the enzyme forms a strong external bond with sildenafil’s pyrimidine ring — effectively mimicking the molecular negotiations observed in solvated crystals. Chemistry, it seems, repeats itself in life and in matter.
Multicomponent Systems: Salts, Cocrystals, and Beyond
The multicomponent forms of sildenafil — salts, cocrystals, and hybrid structures — form the richest and most commercially relevant portion of its solid-state repertoire. The primary motivation for creating these forms is to improve the aqueous solubility and bioavailability of the drug, since the sildenafil free base is sparingly soluble (5–10 mg/L in water).
Sildenafil Citrate: The Iconic Salt
The citrate salt of sildenafil — the form marketed as Viagra — remains the most recognized. Initially thought to be a monohydrate, further studies revealed it to be a non-stoichiometric hemihydrate, with partial water occupancy within its channels. The ambiguity of hydration even puzzled regulatory authorities, as neither Pfizer’s patent filings nor official documentation specified the exact degree of hydration.
Despite this, the citrate salt remains stable and effective, representing a careful balance between manufacturability and therapeutic performance. Dehydration at mild conditions (around 40°C or 0% relative humidity) yields an anhydrous form, though this version is less commonly used due to processing complexities.
Exploring Alternative Salts
The quest for improved solubility and taste has inspired researchers to explore alternative carboxylic acid salts, including fumarate, succinate, glutarate, and oxalate derivatives. Among these, the glutarate salt has shown superior water solubility and bioavailability, nearly doubling the dissolution rate compared to the citrate form.
Such findings underscore how subtle changes in crystal packing can translate into tangible pharmacokinetic advantages. It is a reminder that chemistry at the molecular level directly governs medicine at the patient level.
Hybrid Salt–Cocrystals: The Continuum of Innovation
Between a salt and a cocrystal lies a fascinating continuum — the hybrid salt–cocrystal. These structures exhibit partial proton transfer between the API and the coformer, combining ionic and neutral interactions within a single lattice. This behavior typically arises when the pKa difference between sildenafil and the coformer lies between 0 and 3.
Recent studies have identified sildenafil hybrids with acids like tartaric acid, 3-hydroxybenzoic acid, and 3,4-dihydroxybenzoic acid, resulting in enhanced solubility and stability. Such hybrid forms exemplify the emerging frontier of crystal engineering, where the boundary between salt and cocrystal is intentionally blurred to achieve optimized performance.
Cocrystals for Therapeutic Synergy
Cocrystallization also offers creative possibilities for polypharmacology — pairing sildenafil with coformers that complement its pharmacodynamics. For instance, a sildenafil–acetylsalicylic acid (aspirin) cocrystal has been synthesized to potentially mitigate cardiovascular risks associated with PDE5 inhibition. By combining a vasodilator and an antiplatelet agent in a single crystalline entity, researchers envision dual-action therapeutics that address both sexual health and cardiac safety.
Intellectual Property and Regulatory Considerations
Polymorphism is not merely a scientific curiosity; it is also a regulatory and commercial battlefield. Each unique crystal form of a drug can constitute a separate patentable entity, effectively extending a company’s market exclusivity. Conversely, unexpected polymorphic transitions can invalidate patents or complicate generic production.
For sildenafil, the discovery of over forty distinct solid forms illustrates both opportunity and complexity. The U.S. Food and Drug Administration (FDA) requires applicants introducing new solid forms — whether salts, hydrates, or cocrystals — to demonstrate their structural identity and stability. Key to this is locating the position of labile protons within the crystal lattice, which distinguishes an ionic salt from a neutral cocrystal.
Given the moderate basicity (pKa ≈ 6.8) of sildenafil’s piperazine moiety, the molecule sits precisely at the border between salt and cocrystal formation, further enriching its structural versatility and intellectual intrigue.
The Role of Computational Crystal Engineering
Modern pharmaceutical research increasingly blends experimental crystallography with computational modeling. In sildenafil’s case, molecular electrostatic potential mapping has identified multiple potential hydrogen-bond donors and acceptors, guiding researchers toward promising coformers.
By predicting intermolecular interaction sites, scientists have designed targeted cocrystallization experiments — discovering 23 new solid forms through such hybrid screening. This synergy between computation and experimentation represents the new paradigm in drug design: data-driven, predictive, and efficient.
Moreover, the sildenafil example has helped shape the discipline of Supramolecular Chemistry, where molecular recognition principles guide the assembly of complex architectures. Lessons learned from sildenafil’s solid forms have informed broader strategies for improving the solubility, stability, and manufacturability of other poorly soluble drugs.
The Broader Impact: From Crystals to Clinical Reality
Why should physicians or patients care about polymorphism and cocrystals? The answer lies in performance consistency. Each solid form determines how the drug behaves in the body — how fast it dissolves, how reliably it acts, and how long it lasts.
For sildenafil, small structural differences can mean faster onset of action, improved tolerance, or fewer side effects. Newer solid forms, with higher solubility or better bioavailability, could lead to next-generation formulations — perhaps rapid-dissolving oral films, sublingual tablets, or combined-therapy cocrystals that deliver multifaceted benefits.
Beyond sildenafil itself, this research has advanced our collective understanding of pharmaceutical materials science, showing how molecular architecture underpins therapeutic success. It is not hyperbole to say that every well-performing tablet represents a triumph of chemistry over chaos.
Conclusion: Viagra’s Legacy Beyond the Bedroom
The story of sildenafil is a narrative of serendipity, science, and structural elegance. From its accidental discovery as a failed heart medication to its reinvention as a global symbol of potency, it has continued to surprise — not only in medicine but also in materials science.
Today, with over 40 known crystalline forms, sildenafil stands as a benchmark in solid-state pharmaceutical research. Its polymorphs, solvates, and cocrystals illuminate how a single molecule can adapt across environments, much like the patients it serves — resilient, flexible, and profoundly human.
In essence, the “blue pill” is more than a cultural icon. It is a case study in the art of molecular engineering — a testament to how chemistry, when practiced with curiosity and precision, can transform not just health outcomes but scientific paradigms.
FAQ: Understanding the Solid-State Landscape of Sildenafil
1. Why are there so many solid forms of sildenafil?
Because sildenafil contains multiple functional groups capable of hydrogen bonding and π–π interactions, it readily forms diverse crystalline structures with solvents and coformers. These different arrangements optimize various properties such as solubility, stability, and manufacturability.
2. What is the practical importance of polymorphism in Viagra?
Each polymorph or salt form can influence how quickly the drug dissolves and how effectively it is absorbed. Controlling these forms ensures consistent efficacy, shelf stability, and patient safety — while also providing avenues for patent protection.
3. Could new sildenafil forms improve the drug in the future?
Yes. Novel salts and cocrystals, especially hybrid forms, may enhance solubility and onset of action or combine sildenafil with cardioprotective agents like aspirin. Future formulations could deliver the same therapeutic benefits with fewer side effects and improved pharmacokinetic profiles.