Introduction
It is rare in modern pharmacology for a drug to reinvent itself so dramatically. Yet sildenafil, a molecule initially designed for angina and later repurposed for erectile dysfunction, continues to surprise. Beyond its well-known vasodilatory action through phosphodiesterase type 5 (PDE5) inhibition, accumulating evidence reveals a remarkable anti-tumor potential. Recent studies, including a comprehensive in vivo and in silico investigation using the Ehrlich Ascites Carcinoma (EAC) murine model, have illuminated sildenafil’s ability to induce apoptosis, modulate immune function, and suppress tumor proliferation.
What makes this finding particularly compelling is not only the evidence itself but its translational promise. Sildenafil’s pharmacokinetic profile, safety record, and established clinical infrastructure make it a prime candidate for drug repurposing—the process of finding new therapeutic uses for existing medications. In oncology, where treatment resistance and toxicity remain formidable barriers, a well-characterized, cost-effective, and tolerable drug like sildenafil could offer an unexpected therapeutic edge.
This article explores how sildenafil’s molecular mechanisms intersect with cancer biology, how it potentiates traditional chemotherapy, and how its immunomodulatory effects might reframe the landscape of adjunct cancer therapy.
From Blood Flow to Tumor Biology: Rethinking Sildenafil’s Mechanisms
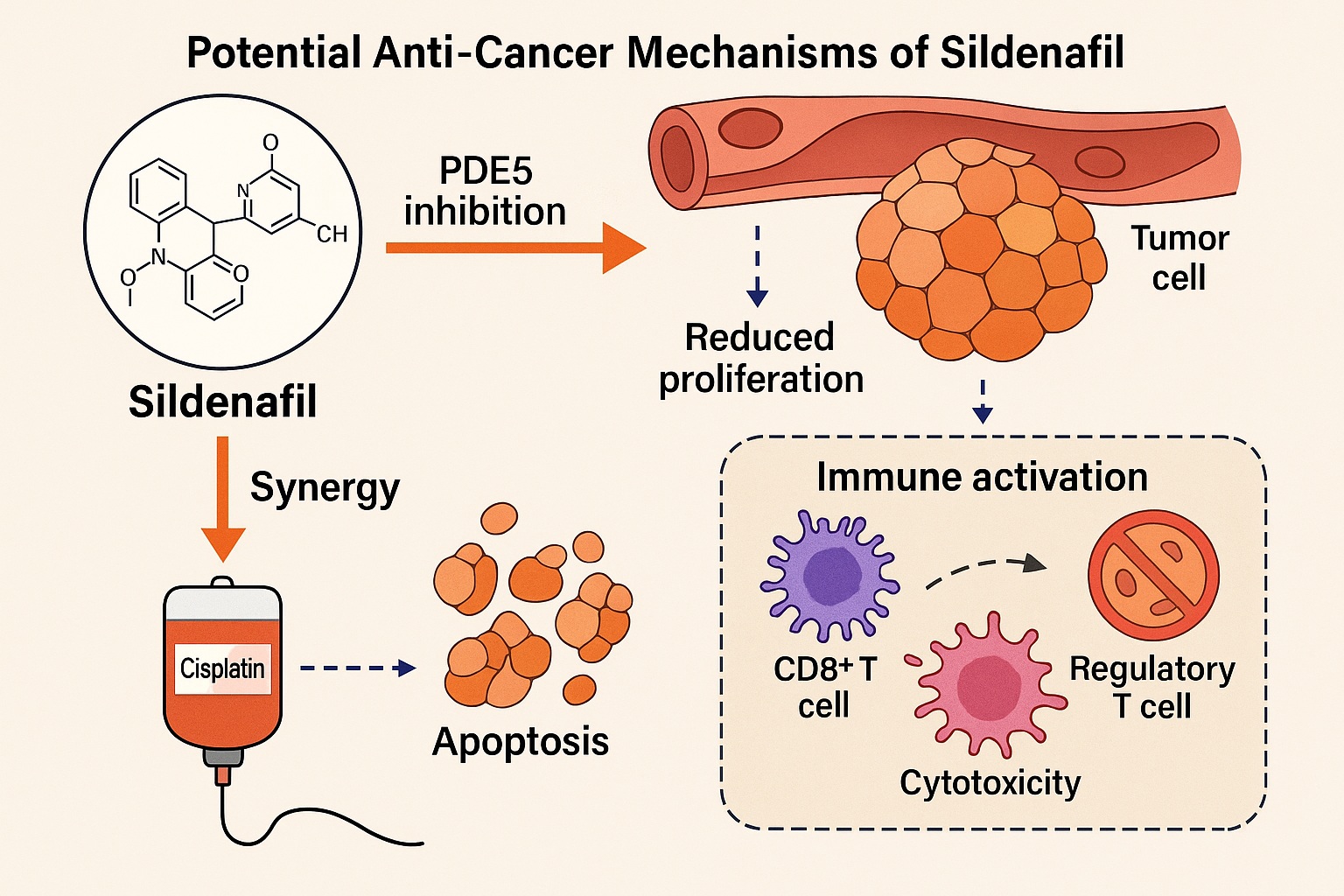
Sildenafil’s primary pharmacological target is PDE5, an enzyme responsible for the degradation of cyclic guanosine monophosphate (cGMP). In vascular smooth muscle, this inhibition leads to cGMP accumulation and subsequent vasodilation. However, the cGMP–PDE5 axis is not confined to blood vessels; it is intricately involved in cell proliferation, apoptosis, and immune signaling within tumor microenvironments.
Tumors, particularly rapidly growing ones such as Ehrlich Ascites Carcinoma, often display overexpression of PDE5. This overactivity dampens cGMP signaling, promoting cellular proliferation, angiogenesis, and survival. By inhibiting PDE5, sildenafil restores intracellular cGMP levels, thereby activating protein kinase G (PKG) pathways that trigger apoptosis and suppress mitogenic signaling cascades such as MAPK and PI3K/Akt.
In other words, sildenafil shifts the balance within tumor cells from survival to programmed death. This mechanistic redirection has far-reaching implications for a range of solid tumors where PDE5 dysregulation contributes to unchecked growth and resistance to therapy.
Beyond direct cytotoxicity, sildenafil enhances tumor perfusion by improving microvascular flow. This effect, paradoxically similar to its erectile function mechanism, may augment the delivery of chemotherapeutic agents to hypoxic tumor cores—regions notoriously resistant to conventional drugs.
Immunomodulation: Awakening the Body’s Intrinsic Defense
Perhaps the most revolutionary aspect of sildenafil’s anti-cancer potential lies in its immunomodulatory role. Tumor cells do not merely proliferate—they actively evade immune surveillance. They accomplish this by recruiting myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs), both of which dampen cytotoxic immune activity.
Sildenafil appears to reverse this immunosuppressive state. In the EAC mouse model, treatment with sildenafil significantly reduced MDSC populations while enhancing CD8+ cytotoxic T-cell infiltration within tumor tissues. The drug’s modulation of nitric oxide and cGMP signaling in immune cells alters cytokine release profiles, increasing interleukin-2 (IL-2) and interferon-gamma (IFN-γ) while suppressing pro-tumorigenic interleukins such as IL-10.
These changes reinvigorate immune-mediated tumor destruction, positioning sildenafil as an immune adjuvant rather than merely a cytostatic agent. In clinical translation, this duality could be transformative: sildenafil may both weaken the tumor’s defenses and fortify the body’s innate offense, a rare combination among small-molecule drugs.
Apoptosis and Antioxidant Defense: The Cellular Balancing Act
Cancer thrives in oxidative chaos. Reactive oxygen species (ROS) act as both signal transducers and genomic saboteurs, fueling mutations and metastasis. Yet excessive oxidative stress can also push cancer cells past the point of repair, leading to apoptosis. Sildenafil appears to exploit this vulnerability with precision.
In vivo assays in EAC-bearing mice revealed that sildenafil significantly elevated antioxidant enzyme activity, including superoxide dismutase (SOD) and catalase, while reducing malondialdehyde (MDA) levels—a marker of lipid peroxidation. At the same time, it upregulated pro-apoptotic proteins such as Bax and caspase-3, while downregulating the anti-apoptotic regulator Bcl-2.
This dual modulation suggests a homeostatic realignment: sildenafil stabilizes oxidative metabolism in normal cells while amplifying oxidative stress selectively within tumor cells, tipping them toward apoptotic death. Such selective toxicity is the holy grail of oncology—destroying the malignant without harming the healthy.
From a molecular perspective, the NO–cGMP–PKG axis again plays a central role. Increased cGMP levels enhance mitochondrial membrane permeability in tumor cells, releasing cytochrome c and activating the caspase cascade. The result is a precise biochemical choreography that culminates in cellular self-destruction.
Synergy with Cisplatin: A Pharmacological Partnership
One of the most compelling translational outcomes of the study lies in sildenafil’s ability to potentiate cisplatin’s anti-tumor effects while mitigating its toxicity. Cisplatin, a cornerstone of chemotherapy, operates through DNA crosslinking but is notorious for its nephrotoxicity and systemic oxidative burden.
In the murine EAC model, combined sildenafil–cisplatin therapy achieved superior tumor suppression compared to cisplatin alone. Importantly, sildenafil co-administration attenuated markers of renal injury such as serum urea and creatinine, indicating a protective antioxidant effect on non-tumor tissues.
Mechanistically, sildenafil appears to sensitize cancer cells to cisplatin by interfering with DNA repair pathways and inhibiting pro-survival kinases. Meanwhile, its vasodilatory and perfusion-enhancing properties likely increase cisplatin delivery to tumor tissues.
This synergy is clinically significant. If replicated in humans, it could mean lower cisplatin doses with equal or greater efficacy, reducing side effects and improving quality of life—an achievement long sought in oncology.
The In Silico Perspective: Molecular Docking and Target Validation
Computational docking analyses provide the molecular rationale for sildenafil’s multi-target effects. The study’s in silico results demonstrated high binding affinity of sildenafil toward proteins involved in both tumor growth and immune signaling, including Lck (lymphocyte-specific protein tyrosine kinase) and MAPK family kinases.
Lck, a critical regulator of T-cell activation, is often dysregulated in cancer, leading to immune escape and tumor persistence. Sildenafil’s stable binding to Lck suggests a direct mechanism by which it enhances T-cell responsiveness and restores immune surveillance. Similarly, docking against MAPK3 and MAPK8 (ERK1 and JNK) revealed interactions consistent with inhibition of pro-survival signaling.
These computational findings support the observed in vivo effects and establish sildenafil as a polypharmacological agent—a drug capable of orchestrating multiple therapeutic effects through interconnected molecular networks.
Translational Implications: The Case for Repurposing Sildenafil in Oncology
The idea of repurposing sildenafil for cancer treatment is not entirely new, but the emerging body of evidence adds both mechanistic clarity and clinical feasibility. Its well-documented pharmacology, oral bioavailability, and favorable safety profile make it an attractive adjunct to existing chemotherapeutic regimens.
Repurposing offers several advantages:
- Accelerated clinical translation: Sildenafil has an established safety dossier, allowing fast-tracked trials.
- Cost-effectiveness: As a generic drug, it offers affordable access for low- and middle-income countries.
- Multi-targeted action: Its combination of vascular, immunologic, and apoptotic mechanisms provides broader therapeutic coverage than single-pathway agents.
Moreover, sildenafil’s ability to normalize tumor vasculature could synergize not only with cytotoxic drugs but also with immunotherapies such as checkpoint inhibitors, potentially enhancing immune cell infiltration into tumor cores. Early preclinical models of melanoma and glioblastoma have already hinted at such benefits.
However, enthusiasm must be tempered with scientific rigor. Translational application requires dose optimization, long-term toxicity studies, and evaluation in human tumor types where PDE5 expression and immune modulation are relevant. Nonetheless, the rationale for clinical trials is now more robust than ever.
Potential Clinical Scenarios: Where Sildenafil Could Fit
If sildenafil’s anticancer properties are confirmed in human studies, several clinical applications become plausible:
- Adjunctive therapy in solid tumors: Co-administration with cisplatin, doxorubicin, or paclitaxel to enhance efficacy and reduce toxicity.
- Immunotherapy sensitization: Used alongside checkpoint inhibitors (e.g., anti-PD-1 or anti-CTLA-4) to reduce immune suppression.
- Tumor microenvironment normalization: Applied in combination with anti-angiogenic therapy to improve perfusion and oxygenation.
These hypothetical uses exemplify a systems pharmacology approach, where sildenafil’s pleiotropic mechanisms complement and amplify established cancer treatments.
Limitations and Future Directions
While the results from the EAC murine model are compelling, translation to human oncology is nontrivial. Differences in tumor microenvironments, immune regulation, and pharmacokinetics may alter sildenafil’s effects. Additionally, chronic administration at higher doses could yield unforeseen cardiovascular or metabolic consequences.
Future research should prioritize:
- Phase I/II clinical trials assessing safety and pharmacodynamics in cancer patients.
- Biomarker studies to identify which tumor types and genotypes respond best.
- Combination therapy trials integrating sildenafil with chemotherapy or immunotherapy protocols.
Equally important will be mechanistic exploration of sildenafil’s interaction with endothelial, immune, and stromal cells in human tumor tissues—data that could refine dosing strategies and maximize therapeutic benefit.
Conclusion
Sildenafil’s evolution from a vasodilator to a potential anti-cancer agent underscores the creativity and power of drug repurposing in modern pharmacology. The findings from the Ehrlich Ascites Carcinoma model reveal a molecule that does far more than relax blood vessels—it orchestrates cellular death, strengthens immune defenses, and enhances chemotherapeutic performance.
In an era of costly cancer drugs and escalating treatment complexity, sildenafil represents a paradoxical innovation: a familiar, affordable compound reimagined through the lens of molecular oncology.
If future clinical trials confirm its efficacy, sildenafil could find a new life not in romantic encounters, but in the battle against malignancy—a transition as unexpected as it is scientifically justified.
FAQ: Sildenafil and Cancer Therapy
1. Can sildenafil really be used as a cancer treatment?
Current evidence supports sildenafil’s anti-tumor effects in animal and cell models. While human trials are needed, its mechanisms—immune activation, apoptosis induction, and synergy with chemotherapy—make it a strong candidate for clinical repurposing.
2. Is it safe to take sildenafil during chemotherapy?
Preclinical data suggest that sildenafil may protect against chemotherapy-induced toxicity, but human dosing and safety profiles must be rigorously established before medical use in oncology.
3. Which cancers might benefit most from sildenafil?
Tumors with high PDE5 expression—such as breast, colon, and lung cancers—are theoretically the most responsive. Ongoing research aims to identify precise genetic and molecular predictors of response.