Introduction
Pulmonary arterial hypertension (PAH) is a devastating condition characterized by progressive increases in pulmonary vascular resistance, ultimately leading to right ventricular failure and premature death. When PAH develops secondary to chronic obstructive pulmonary disease (COPD), the prognosis becomes even grimmer. The dual burden of impaired gas exchange and elevated pulmonary pressures shortens survival and dramatically impairs quality of life.
Current therapeutic strategies remain limited. Traditional COPD management—bronchodilators, corticosteroids, supplemental oxygen—addresses airflow limitation but only partially alleviates pulmonary hypertension. Meanwhile, PAH-specific drugs such as prostacyclin analogues, endothelin receptor antagonists, and phosphodiesterase type 5 (PDE5) inhibitors have reshaped the treatment of idiopathic PAH, yet their role in secondary PAH has remained controversial.
Among these, sildenafil citrate, best known as a therapy for erectile dysfunction, has emerged as a promising candidate. Its pharmacological target—PDE5, highly expressed in the pulmonary vasculature—makes it uniquely positioned to modulate pulmonary vascular tone through enhanced nitric oxide–cGMP signaling. The study conducted by Alkhayat and Eid (2016) explored whether sildenafil could improve hemodynamics and functional capacity in patients with secondary PAH due to COPD.
Pathophysiological Rationale
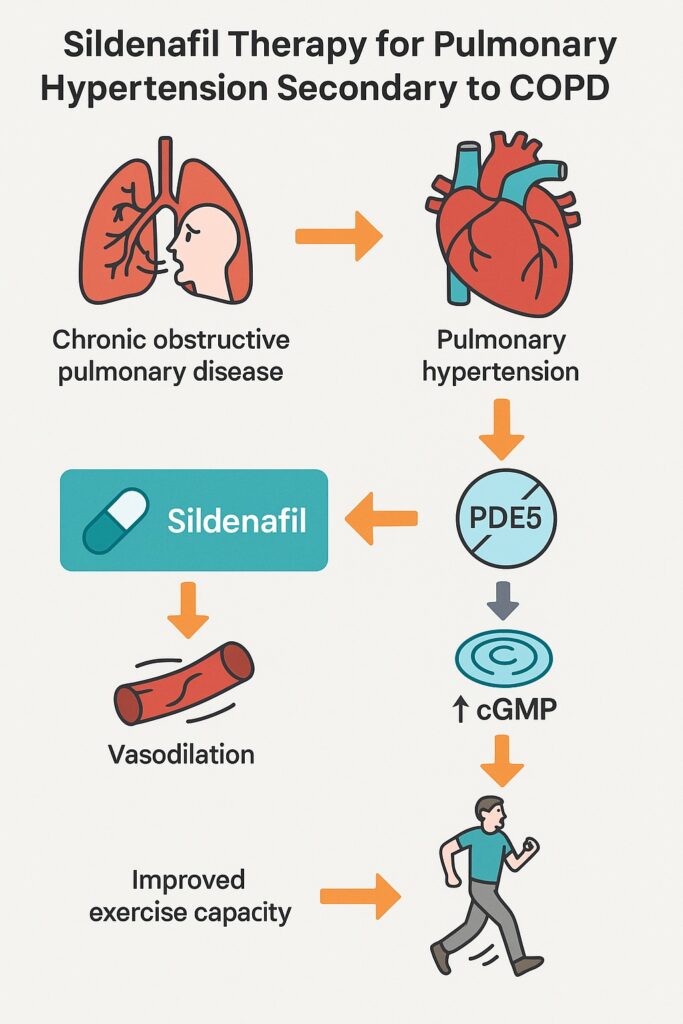
The pulmonary circulation in COPD is exposed to chronic hypoxemia, inflammation, and vascular remodeling. Endothelial dysfunction develops, characterized by decreased bioavailability of vasodilators such as nitric oxide (NO) and prostacyclin, coupled with increased expression of potent vasoconstrictors such as endothelin-1. The result is progressive narrowing and stiffening of pulmonary arteries.
The NO–cGMP pathway is central to vasodilation. NO activates guanylyl cyclase, increasing cGMP within vascular smooth muscle cells. This promotes relaxation and inhibits proliferation. PDE5 rapidly hydrolyzes cGMP, terminating the signal. In PAH, PDE5 expression is upregulated, exaggerating cGMP breakdown and blunting vasodilatory signaling.
By selectively inhibiting PDE5, sildenafil restores cGMP accumulation, amplifying the effects of NO. Beyond vasodilation, cGMP has antiproliferative properties, potentially counteracting vascular remodeling. These mechanistic insights justified the clinical investigation of sildenafil in COPD-associated PAH.
Study Design: A Pragmatic Clinical Trial
Alkhayat and Eid conducted a prospective, placebo-controlled trial involving 139 patients with symptomatic PAH secondary to COPD. Patients were randomized to receive:
- Sildenafil group: 20 mg orally three times daily, in addition to conventional COPD therapy.
- Placebo group: placebo tablets plus standard COPD management.
The treatment duration was 12 weeks.
Primary endpoint: Change in exercise capacity, measured by the six-minute walking test (6MWT).
Secondary endpoints:
- Change in mean pulmonary artery pressure (mPAP) measured by echocardiography.
- Incidence of clinical worsening (death, hospitalization, or need for additional PAH therapy).
- Safety and tolerability, assessed through clinical and laboratory monitoring.
Results: Sildenafil Shows Clear Benefits
Exercise Capacity
Patients receiving sildenafil exhibited significant improvements in walking distance:
- +51 meters placebo-corrected improvement at 12 weeks (p < 0.001).
- Gains were apparent by week 4 and sustained through week 12.
To the untrained eye, 51 meters may sound modest. Yet in PAH trials, even a 30–40 meter increase in 6MWT is clinically meaningful, translating into improved ability to perform daily activities.
Hemodynamics
Echocardiographic assessment showed:
- Significant reduction in mean pulmonary artery pressure in the sildenafil group (p = 0.04).
- Placebo patients demonstrated no meaningful change.
Clinical Worsening
The incidence of clinical worsening did not differ significantly between groups, but the study was underpowered to assess mortality or long-term outcomes.
Safety
Adverse events were mostly mild and included:
- Headache
- Flushing
- Dyspepsia
- Diarrhea
No deaths were attributed to sildenafil. Compared with other PAH agents (e.g., intravenous prostacyclin with risks of infection, or bosentan with hepatotoxicity), sildenafil demonstrated a favorable safety profile.
Discussion: Contextualizing the Findings
The observed 51-meter improvement in 6MWT with sildenafil parallels results achieved by established PAH therapies:
- Intravenous epoprostenol: ~47 m gain
- Inhaled iloprost: ~36 m
- Oral bosentan: ~44 m
- Subcutaneous treprostinil: ~16 m
Thus, sildenafil not only works but matches or outperforms other agents in functional capacity improvements—without the logistical burdens of pumps or inhalation systems.
Hemodynamic improvements reinforce its utility. While echocardiography lacks the precision of right heart catheterization, reductions in mPAP are consistent with sildenafil’s known pharmacodynamics.
Limitations
Despite encouraging findings, several caveats warrant mention:
- Short duration (12 weeks): Long-term efficacy, safety, and survival impact remain unknown.
- Indirect mPAP measurement: Right heart catheterization is the gold standard but was not used.
- Exclusion criteria: Patients with PAH due to other etiologies (HIV, portal hypertension, congenital heart disease) were excluded, limiting generalizability.
- Clinical worsening endpoint: Low overall incidence and short follow-up precluded meaningful conclusions.
Nonetheless, the trial demonstrates proof-of-concept efficacy and safety in COPD-associated PAH.
Clinical Implications
For clinicians managing patients with COPD complicated by secondary PAH, sildenafil offers:
- Improved exercise tolerance, allowing patients to regain functional independence.
- Reduction in pulmonary pressures, potentially delaying right heart failure progression.
- Oral administration, a significant advantage over parenteral prostanoids.
However, sildenafil should not be viewed as a cure. COPD-associated PAH is multifactorial, and comprehensive care—oxygen therapy, pulmonary rehabilitation, optimization of COPD therapy—remains essential. Sildenafil represents an adjunct, not a replacement.
Conclusion
The study by Alkhayat and Eid adds to the growing evidence that sildenafil citrate is an effective and safe therapy for PAH secondary to COPD. By enhancing cGMP signaling, sildenafil improves both exercise capacity and pulmonary hemodynamics.
While long-term survival data are lacking, the symptomatic and functional improvements are undeniable. For a patient population often left with few options, sildenafil represents a beacon of hope—an inexpensive, well-tolerated therapy that bridges the gap between COPD and pulmonary hypertension care.
FAQ
1. Can sildenafil be used in all COPD patients?
No. It is specifically indicated for patients with documented pulmonary arterial hypertension secondary to COPD. Patients without PAH do not benefit from PDE5 inhibition in terms of lung function.
2. Is sildenafil safe for long-term use in PAH?
The study confirmed safety over 12 weeks. Longer-term studies are needed, but sildenafil has been widely used in idiopathic PAH with acceptable safety profiles. Monitoring is still necessary.
3. How does sildenafil compare with other PAH therapies?
Sildenafil improves exercise capacity to a degree comparable to prostanoids and endothelin receptor antagonists, with fewer logistical and safety concerns. Its oral availability and tolerability make it an attractive first-line adjunct in selected patients.