Introduction
Few topics in men’s health inspire as much discussion as the intersection of hormones, sexual function, and the prostate. Testosterone, long regarded as the engine of male physiology, exerts wide-reaching effects on muscle mass, mood, libido, and the cardiovascular system. Yet its darker side manifests in the prostate gland, where excessive androgen stimulation drives hyperplasia, urinary obstruction, and — in more ominous cases — malignant transformation.
Sildenafil citrate, the first widely adopted phosphodiesterase-5 (PDE-5) inhibitor, revolutionized erectile dysfunction treatment. By amplifying nitric oxide (NO)-mediated vasodilation, sildenafil restores erectile function in countless men. But its pharmacological effects extend far beyond penile tissue. The enzyme PDE-5 is expressed in vascular smooth muscle, pulmonary arteries, myocardium, and, importantly, the urogenital tract.
The study at hand examines a question that has hovered in the background of urology for years: what happens when sildenafil interacts with testosterone in the prostate? More specifically, can sildenafil influence testosterone-induced prostate hypertrophy and modify the tone of the urinary bladder neck, the muscular gateway that controls urine outflow?
This question is not trivial. Benign prostatic hyperplasia (BPH) affects nearly half of men over the age of 50 and represents one of the leading causes of lower urinary tract symptoms (LUTS). Treatments, from alpha-blockers to 5-alpha-reductase inhibitors, often come with side effects. The idea that sildenafil — a drug already embraced for erectile dysfunction — might simultaneously ease urinary obstruction and mitigate androgen-driven prostate enlargement is enticing.
In this article, we will dissect the study’s findings and expand upon them to explore broader clinical implications. Along the way, we will revisit the biology of testosterone, the pathophysiology of BPH, and the unexpected reach of PDE-5 inhibitors in urology.
Testosterone and the Prostate: A Delicate Balance
Testosterone’s role in the prostate is both essential and hazardous. Under normal physiology, testosterone is converted locally into dihydrotestosterone (DHT) by the enzyme 5-alpha-reductase. DHT binds androgen receptors with greater affinity than testosterone itself, stimulating prostate growth and maintaining glandular function.
This arrangement ensures reproductive viability during the prime years of life. Yet, as men age, the same androgenic influence contributes to uncontrolled proliferation of prostate tissue. The result is benign prostatic hyperplasia, a condition characterized by increased glandular mass, stromal remodeling, and compression of the urethra.
The relationship between testosterone therapy and prostate growth is especially important today, given the widespread prescription of testosterone replacement therapy (TRT). While TRT offers undeniable benefits to hypogonadal men, clinicians must remain vigilant about its potential to accelerate prostate enlargement or worsen LUTS.
The animal model in the study employed exogenous testosterone administration to reliably induce prostate hypertrophy, simulating the hormonal conditions that predispose men to BPH. Into this system, sildenafil was introduced — not as an erectile aid, but as a potential modulator of prostate and bladder physiology.
PDE-5 Inhibition Beyond Erectile Function
The mechanism of sildenafil deserves closer inspection. PDE-5 is the enzyme responsible for degrading cyclic guanosine monophosphate (cGMP), the intracellular messenger that mediates nitric oxide signaling. By inhibiting PDE-5, sildenafil prolongs cGMP activity, producing sustained smooth muscle relaxation and vasodilation.
In penile tissue, this translates into enhanced blood engorgement and erection. Yet PDE-5 expression is not confined to the corpus cavernosum. It is present in vascular beds throughout the body and, critically, in the prostate and bladder neck.
This anatomical distribution implies that sildenafil could reduce smooth muscle tone in the lower urinary tract, much like alpha-blockers currently prescribed for LUTS. Unlike alpha-blockers, which target adrenergic signaling, sildenafil acts through the NO-cGMP pathway, potentially offering symptom relief without the same profile of side effects.
Additionally, sildenafil has been shown in some contexts to exert anti-proliferative and anti-fibrotic effects, raising the question of whether it could counteract testosterone-induced tissue overgrowth in the prostate.
Study Design: Investigating Hormone-Drug Interactions
The researchers utilized an animal model where testosterone was administered to induce prostate hypertrophy, mimicking androgen-driven enlargement. Sildenafil was then given to assess whether it altered prostate growth and to evaluate its effects on bladder neck muscle tone.
The primary outcomes included:
- Prostate weight and histology, serving as markers of hypertrophy.
- Contractile responses of the bladder neck muscle, measured in vitro to assess relaxation.
- Molecular analysis of PDE-5 expression, offering mechanistic insight.
By combining morphological, physiological, and biochemical endpoints, the investigators sought a comprehensive picture of sildenafil’s influence in this context.
Key Findings: The Dual Nature of Sildenafil
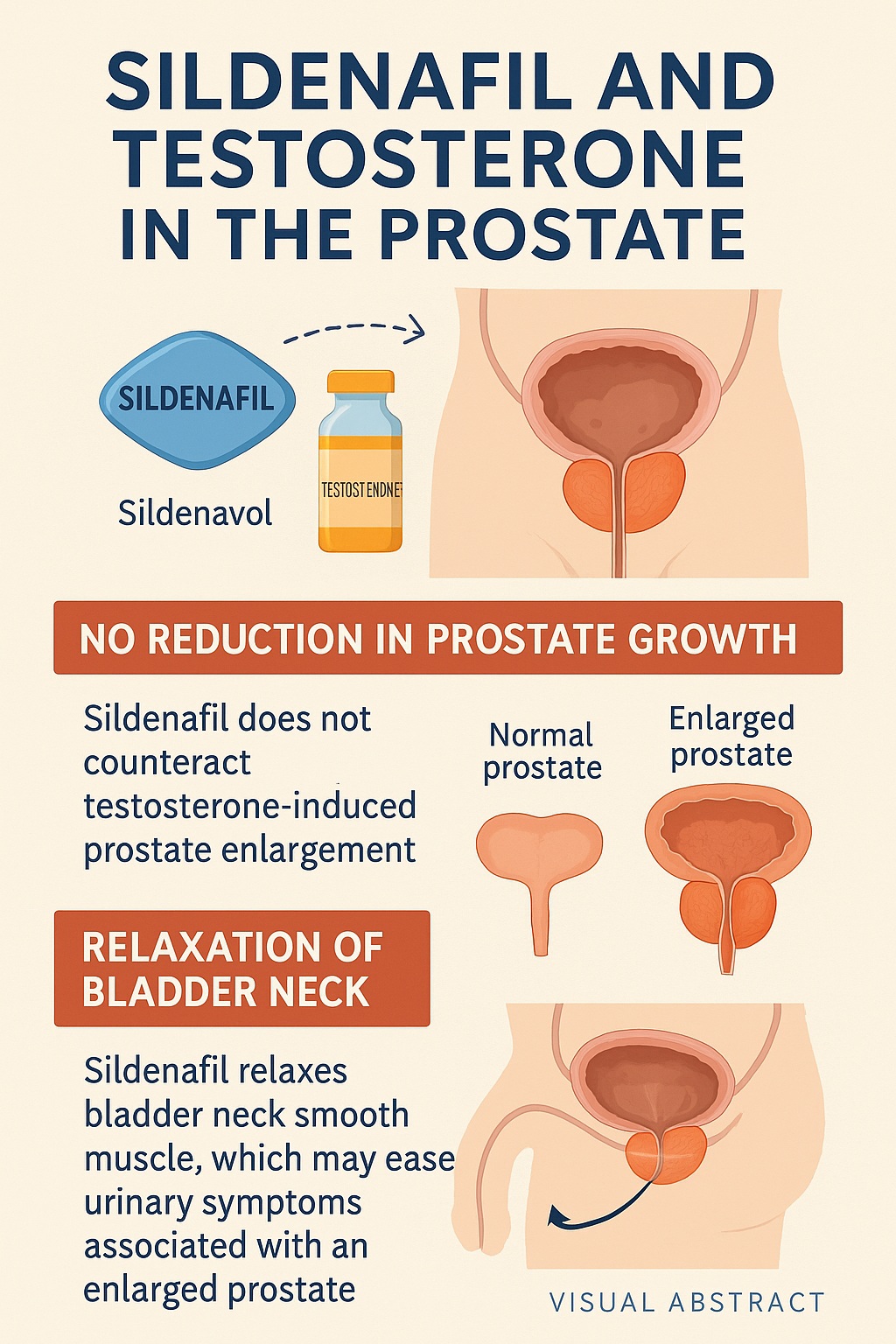
The results revealed a nuanced picture. Sildenafil did not prevent testosterone-induced prostate hypertrophy. Prostate weights in testosterone-treated animals remained elevated regardless of sildenafil administration, indicating that PDE-5 inhibition alone cannot block androgen-driven proliferation.
However, when bladder neck tissues were examined, sildenafil produced significant smooth muscle relaxation. This effect was mediated via the NO-cGMP pathway, consistent with sildenafil’s known pharmacology. By reducing bladder outlet resistance, sildenafil could theoretically improve urinary flow even in the presence of an enlarged prostate.
Thus, the study delivered a clear message: sildenafil does not halt prostate growth, but it does relax bladder neck muscles, potentially relieving obstructive symptoms.
Clinical Implications for Benign Prostatic Hyperplasia
What do these findings mean for men suffering from LUTS due to BPH? The most immediate implication is that sildenafil may offer symptomatic relief without altering the underlying disease process. In other words, while it does not shrink the prostate, it may reduce the functional obstruction at the bladder outlet.
This effect mirrors the therapeutic action of alpha-blockers, which relax smooth muscle tone in the prostate and bladder neck to improve urinary flow. Yet sildenafil’s mechanism, through cGMP rather than adrenergic blockade, may offer unique advantages, particularly for men who also experience erectile dysfunction.
Indeed, clinical trials have shown that PDE-5 inhibitors improve International Prostate Symptom Scores (IPSS) in men with LUTS. For many patients, this dual benefit — improved erections and better urination — represents a compelling therapeutic option. The current findings provide mechanistic support for these clinical observations.
That said, sildenafil cannot replace disease-modifying therapies such as 5-alpha-reductase inhibitors, which directly reduce prostate volume by inhibiting DHT synthesis. For men with significantly enlarged prostates or progressive disease, combination approaches may remain necessary.
The Biology of the Bladder Neck
The bladder neck serves as a muscular gateway between the bladder and urethra. Its tone is tightly regulated by autonomic signaling and contributes substantially to urinary resistance. In BPH, even modest increases in tone can exacerbate obstruction caused by enlarged prostatic tissue.
By relaxing bladder neck smooth muscle, sildenafil reduces this resistance and promotes more efficient voiding. The NO-cGMP pathway is a natural modulator of bladder contractility, and PDE-5 inhibition amplifies its influence. This provides a plausible explanation for clinical improvements in urinary flow seen in men treated with PDE-5 inhibitors.
It also suggests that the therapeutic benefits of sildenafil in LUTS extend beyond the prostate itself, highlighting the importance of functional obstruction at the bladder outlet.
Synergy with Testosterone: A Double-Edged Sword
One might ask: if sildenafil relaxes the bladder neck, could it offset the hypertrophic effects of testosterone therapy in men receiving TRT? The study provides a cautious answer. While sildenafil may relieve obstructive symptoms, it does not prevent testosterone-induced prostate growth.
This distinction is critical in clinical practice. Men on TRT may experience symptomatic relief with sildenafil, but they remain at risk for prostate enlargement and its long-term consequences. Regular monitoring of prostate size, prostate-specific antigen (PSA) levels, and urinary symptoms remains essential.
In fact, there is a subtle irony here: testosterone promotes growth, sildenafil eases flow, but the fundamental imbalance persists. The combination may mask symptoms while structural changes progress unnoticed — a reminder that symptom relief does not always equate to disease modification.
Broader Pharmacological Considerations
The study also highlights the broader pharmacological footprint of sildenafil. Beyond erectile dysfunction, PDE-5 inhibitors are being studied for pulmonary hypertension, heart failure, Raynaud’s phenomenon, and even certain fibrotic diseases. Their actions on smooth muscle, endothelial function, and cellular signaling pathways grant them an unusually wide therapeutic reach.
In urology, sildenafil and related agents such as tadalafil and vardenafil have already secured a place in the treatment of LUTS, often in combination with alpha-blockers or 5-alpha-reductase inhibitors. The current findings reinforce their utility and provide mechanistic clarity.
It is worth noting, however, that PDE-5 inhibitors are not free of side effects. Headache, flushing, nasal congestion, and systemic hypotension remain common. Clinicians must also exercise caution in men taking nitrates, where the risk of profound hypotension is significant.
Future Research Directions
Several avenues for future investigation arise from this study. First, the precise molecular pathways by which sildenafil influences bladder neck tone warrant deeper exploration. Identifying downstream targets of cGMP signaling in smooth muscle could reveal novel therapeutic strategies.
Second, the potential for combination therapies merits attention. Could sildenafil be paired with 5-alpha-reductase inhibitors to address both functional obstruction and structural growth? Might such combinations provide superior symptom relief while slowing disease progression?
Third, sex hormone interactions deserve continued scrutiny. Understanding how androgens and PDE-5 inhibitors interact at the molecular level could clarify why some patients respond better than others.
Finally, long-term clinical trials are needed to confirm whether the symptomatic improvements observed with sildenafil translate into meaningful outcomes such as reduced risk of urinary retention, decreased need for surgery, or improved quality of life.
Conclusion
The relationship between testosterone, sildenafil, and the prostate is complex, reflecting the intricate interplay of hormones, smooth muscle physiology, and pharmacology. The study under review makes one point clear: sildenafil does not prevent testosterone-induced prostate hypertrophy. Androgen-driven growth marches on, undeterred by PDE-5 inhibition.
Yet, all is not lost. By relaxing bladder neck smooth muscle, sildenafil offers symptomatic relief that could significantly improve quality of life for men with LUTS. For patients who simultaneously suffer from erectile dysfunction, the dual benefit is particularly attractive.
Ultimately, sildenafil should not be regarded as a disease-modifying therapy for BPH, but rather as a functional ally — one that eases the burden of obstruction without rewriting the biology of prostate growth. The distinction is subtle but critical, reminding clinicians and patients alike that relief and cure are not always synonymous.
As research advances, we may discover new ways to harness the NO-cGMP pathway for urologic health. Until then, sildenafil remains a versatile, if imperfect, tool in the urologist’s armamentarium.
FAQ
1. Does sildenafil shrink the prostate?
No. Sildenafil does not reduce prostate size. It works by relaxing bladder neck smooth muscle, improving urinary flow, but it does not counteract testosterone-driven prostate growth.
2. Can men on testosterone replacement therapy use sildenafil safely?
Yes, but with caveats. Sildenafil can improve urinary symptoms and erectile function, but men on TRT remain at risk for prostate enlargement. Regular monitoring of prostate health is essential.
3. Is sildenafil a replacement for standard BPH medications?
Not entirely. While sildenafil can improve symptoms, it does not alter disease progression. Many men may require additional therapies such as alpha-blockers or 5-alpha-reductase inhibitors, depending on prostate size and severity of symptoms.