Introduction
Cardiovascular disease remains the leading cause of death in individuals with diabetes, and among its many manifestations, diabetic cardiomyopathy holds a particularly sinister reputation. Defined as structural and functional abnormalities of the myocardium in the absence of overt ischemia or hypertension, it represents one of the most elusive clinical entities in modern cardiology. Its progression is slow, its diagnosis often delayed, and its therapeutic options frustratingly limited.
The complexity of diabetic cardiomyopathy lies not only in hyperglycemia-induced injury but also in a cascade of downstream disruptions. Oxidative stress, inflammation, mitochondrial dysfunction, and impaired calcium handling converge to produce maladaptive remodeling of the heart. For decades, clinicians and researchers have tried to tame this beast, often with partial success.
Yet new therapeutic candidates continue to emerge, some from unexpected corners of pharmacology. Among them are sildenafil, better known for its role in erectile dysfunction; testosterone, long debated in cardiovascular endocrinology; and an intriguing compound, fructose diphosphate strontium (FDP-Sr), a metabolic modulator. Their convergence in the study of diabetic cardiomyopathy highlights the evolving landscape of translational medicine, where molecules repurposed from one indication may unlock entirely new therapeutic vistas.
The Burden of Diabetic Cardiomyopathy
To appreciate why new therapies matter, one must first grasp the clinical and biological burden of diabetic cardiomyopathy. Diabetes creates a hostile myocardial environment. Hyperglycemia initiates glycation of proteins, stiffening the myocardium and impairing compliance. Lipotoxicity adds to the insult, depositing excess fatty acids in cardiac tissue. Microvascular disease further restricts oxygen delivery, compounding metabolic stress.
What differentiates diabetic cardiomyopathy from other forms of heart failure is its predilection for subtle diastolic dysfunction early in the disease course. Patients may present with preserved ejection fraction yet report symptoms of exertional dyspnea and fatigue. As the disease progresses, systolic impairment emerges, culminating in overt heart failure.
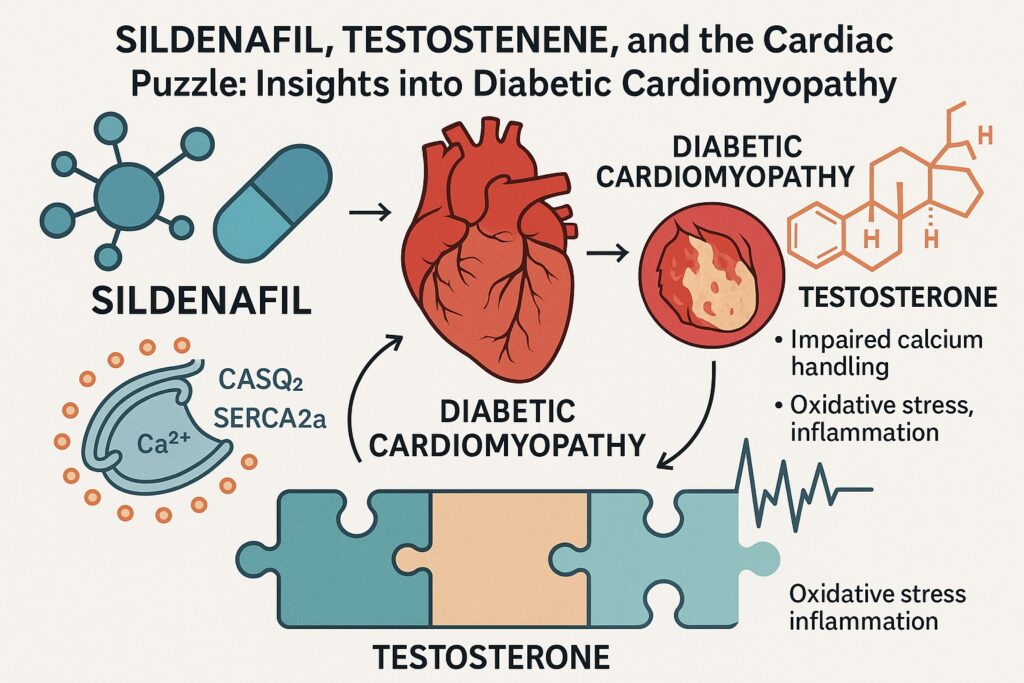
The molecular hallmarks are equally striking. Calcium-handling proteins such as sarco/endoplasmic reticulum Ca²⁺-ATPase (SERCA2a), calsequestrin 2 (CASQ2), and the ryanodine receptor stabilizer FKBP12.6 often demonstrate dysregulation. These alterations disturb excitation–contraction coupling, rendering cardiomyocytes less capable of precise calcium cycling. The result is impaired relaxation, contractile inefficiency, and vulnerability to arrhythmia.
Conventional therapies—angiotensin-converting enzyme inhibitors, beta-blockers, and diuretics—address the hemodynamic consequences but rarely reverse the underlying pathology. Hence, the search for targeted interventions that restore cellular homeostasis rather than merely alleviating symptoms.
Sildenafil: Beyond Vasodilation
Sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, has long been celebrated (or marketed) for its role in erectile dysfunction. Its pharmacological foundation is simple: by inhibiting PDE5, it prevents the breakdown of cyclic guanosine monophosphate (cGMP), enhancing nitric oxide (NO)-mediated vasodilation.
The cardiac relevance of sildenafil was initially underappreciated, but subsequent research revealed that PDE5 is expressed in cardiomyocytes. In pathological states such as hypertrophy, ischemia, and diabetes, its expression is upregulated. By inhibiting PDE5, sildenafil increases cGMP levels, activating protein kinase G (PKG), which in turn modulates calcium handling, reduces hypertrophy, and dampens oxidative stress.
In diabetic cardiomyopathy models, sildenafil has shown several beneficial effects:
- Improved left ventricular function by enhancing relaxation.
- Reduced oxidative stress via NO–cGMP–PKG signaling.
- Stabilization of calcium-handling proteins, preserving contractile efficiency.
One could say sildenafil’s cardiac career was an “accidental promotion”—from bedroom to cardiology ward. Yet, unlike many repurposing anecdotes, this one is backed by a coherent mechanistic rationale and consistent preclinical evidence.
Testosterone: The Endocrine Enigma
Testosterone and the cardiovascular system have always shared a complicated relationship. On one hand, hypogonadism in men is associated with increased cardiovascular risk, insulin resistance, and reduced quality of life. On the other, supraphysiological testosterone use—particularly in anabolic steroid abuse—clearly increases cardiovascular morbidity. The middle ground is where science wrestles with nuance.
In the diabetic myocardium, testosterone plays several roles. Physiological replacement has been linked to improved glucose metabolism, reduced fat mass, and enhanced vascular function. More specifically, testosterone appears to modulate calcium handling in cardiomyocytes. It influences the expression of SERCA2a and CASQ2, proteins that restore calcium to the sarcoplasmic reticulum during diastole and maintain appropriate buffering capacity.
Deficiency in testosterone may therefore exacerbate diastolic dysfunction and oxidative stress in diabetic hearts. Replacement therapy, if judiciously applied, has the potential to restore not only endocrine balance but also myocardial resilience. Still, cardiologists approach this intervention with warranted caution, for the boundary between therapeutic benefit and cardiovascular risk is thin.
FDP-Sr: A Metabolic Ally
If sildenafil and testosterone are well-known names, FDP-Sr might sound like an obscure guest at the pharmacological table. Fructose-1,6-diphosphate (FDP) is a glycolytic intermediate that supports anaerobic metabolism. Strontium, meanwhile, has garnered interest in bone health for its effects on osteoblasts and osteoclasts. When combined, FDP-Sr offers intriguing possibilities for myocardial energy metabolism.
In diabetic cardiomyopathy, where mitochondrial efficiency is compromised, FDP-Sr provides an alternative energy source. It enhances glycolysis, reduces reliance on fatty acid oxidation, and attenuates reactive oxygen species generation. Moreover, its influence on calcium handling proteins such as CASQ2 and SERCA2a aligns it with the broader therapeutic goals of restoring excitation–contraction coupling.
Though less glamorous than sildenafil or testosterone, FDP-Sr represents the unsung hero—supporting the heart’s metabolic needs when traditional pathways falter. Its translational journey remains early, but its mechanistic rationale deserves continued exploration.
Calcium-Handling Proteins: The Molecular Orchestra
Central to diabetic cardiomyopathy is the disruption of calcium homeostasis. Like a poorly tuned orchestra, cardiomyocytes struggle to coordinate contraction and relaxation. Three proteins deserve special mention.
SERCA2a: This ATP-driven pump sequesters calcium back into the sarcoplasmic reticulum during diastole. Reduced SERCA2a expression or activity prolongs cytosolic calcium transients, impairing relaxation and fostering diastolic dysfunction.
CASQ2: As the major calcium-binding protein in the sarcoplasmic reticulum, calsequestrin 2 ensures a reservoir of releasable calcium. Dysregulation leads to unstable calcium release, arrhythmogenesis, and contractile inefficiency.
FKBP12.6: This small immunophilin stabilizes the ryanodine receptor (RyR2), preventing spontaneous calcium leak. Reduced FKBP12.6 expression destabilizes RyR2, leading to diastolic calcium leak and arrhythmic vulnerability.
In diabetic cardiomyopathy, all three are compromised. The cumulative effect is a heart that cannot relax, cannot contract efficiently, and is prone to electrical chaos. The therapeutic strategies discussed—sildenafil, testosterone, FDP-Sr—converge on restoring harmony to this orchestra.
Oxidative Stress and Inflammation: Twin Antagonists
If calcium dysregulation is the orchestra, oxidative stress and inflammation are the unruly audience members disrupting the performance. Diabetes creates a milieu rich in reactive oxygen species (ROS). Hyperglycemia drives mitochondrial overproduction of superoxide, while advanced glycation end products (AGEs) amplify the inflammatory response.
Oxidative stress damages proteins, lipids, and DNA, while inflammation recruits cytokines that exacerbate myocardial fibrosis and apoptosis. Together, they accelerate structural remodeling of the heart.
Therapeutics that attenuate these antagonists provide meaningful benefit. Sildenafil’s activation of PKG reduces oxidative stress. Testosterone replacement, by improving metabolic parameters, indirectly dampens inflammatory signals. FDP-Sr, by enhancing glycolysis, reduces ROS generation at its mitochondrial source. The common thread is not a complete silencing of these antagonists but rather a reduction in their disruptive volume, allowing the orchestra of calcium handling to perform with greater fidelity.
Integrating the Therapeutic Triad
What happens when sildenafil, testosterone, and FDP-Sr are combined in experimental models? Preliminary evidence suggests their effects are synergistic. Sildenafil enhances NO–cGMP signaling, testosterone restores calcium-handling protein expression, and FDP-Sr supplies metabolic resilience. Together, they address multiple facets of diabetic cardiomyopathy: contractile dysfunction, oxidative stress, inflammation, and energy deficiency.
This integration illustrates a key principle in modern pharmacotherapy: complex diseases demand multipronged solutions. No single drug can fully reverse the myriad insults of diabetes on the heart. Combination strategies, when rationally designed, can offer a more comprehensive approach. Of course, translating such combinations into clinical practice will require careful evaluation of safety, dosing, and interactions.
Translational Challenges
While preclinical data are promising, several challenges temper enthusiasm. First, animal models of diabetic cardiomyopathy, though informative, never fully replicate human disease. Mice do not live long enough, nor do their metabolic profiles perfectly mimic those of patients with type 2 diabetes.
Second, the cardiovascular safety of testosterone remains controversial. Though physiological replacement appears safe in hypogonadal men, concerns about thrombosis, prostate disease, and long-term cardiovascular outcomes persist.
Third, FDP-Sr remains largely experimental, with limited clinical data. Its metabolic benefits are attractive, but strontium’s effects on bone and mineral metabolism raise questions about long-term use.
Finally, combination therapy is inherently more complex than monotherapy. Regulatory pathways, clinical trial design, and patient adherence all become more challenging. Yet these hurdles are not insurmountable; they simply remind us that translational medicine is a marathon, not a sprint.
Clinical Implications and Future Directions
Despite the challenges, the therapeutic insights gained from sildenafil, testosterone, and FDP-Sr are substantial. They emphasize the importance of targeting calcium-handling proteins, oxidative stress, and metabolism in diabetic cardiomyopathy.
Future directions include:
- Developing more selective PDE5 inhibitors with cardiac-specific effects.
- Refining testosterone replacement strategies to maximize benefit and minimize risk.
- Exploring metabolic modulators like FDP-Sr in clinical trials, perhaps as adjuncts to established heart failure therapies.
Moreover, the story underscores the value of drug repurposing. Sildenafil’s journey from angina to erectile dysfunction to cardiomyopathy highlights the serendipitous nature of pharmacology. Testosterone, long maligned or celebrated depending on the context, may yet prove a valuable ally in diabetic hearts. FDP-Sr, though less known, exemplifies the creative exploration of metabolic interventions.
Conclusion
Diabetic cardiomyopathy remains one of the most formidable challenges in cardiology, a disorder born of metabolic chaos and sustained by molecular disruption. The therapeutic triad of sildenafil, testosterone, and FDP-Sr offers a glimpse into a future where interventions are not limited to symptom management but instead target the underlying biology.
Restoring calcium-handling proteins, reducing oxidative stress, and supporting metabolic resilience represent not only mechanistic elegance but also clinical necessity. Whether these insights will translate into routine practice remains uncertain, but they undeniably enrich our understanding of the diabetic heart.
In the end, the lesson is clear: the heart, though battered by diabetes, can still respond to interventions that respect its biology. And sometimes, the most effective allies are those least expected—from a drug of sexual health fame to a hormone of contested reputation to a metabolic molecule waiting for its moment.
FAQ
1. Why is calcium handling so important in diabetic cardiomyopathy?
Calcium cycling regulates contraction and relaxation of cardiomyocytes. In diabetes, proteins like SERCA2a, CASQ2, and FKBP12.6 are impaired, leading to diastolic dysfunction, systolic weakness, and arrhythmia risk. Restoring calcium homeostasis is therefore central to therapy.
2. Is sildenafil already used in patients with diabetic cardiomyopathy?
Not routinely. While preclinical studies are promising, sildenafil is not currently approved for cardiomyopathy. Its established roles remain in erectile dysfunction and pulmonary hypertension. Clinical trials will be needed to clarify its cardiovascular applications in diabetes.
3. What makes combination therapy attractive in this setting?
Diabetic cardiomyopathy arises from multiple pathological mechanisms—oxidative stress, calcium dysregulation, metabolic derangement. Combination therapy allows simultaneous targeting of these pathways. Sildenafil, testosterone, and FDP-Sr together exemplify this multipronged approach.