Introduction
Erectile dysfunction (ED) in men with type 2 diabetes mellitus (T2DM) is not simply a urological nuisance—it is a multifaceted complication that reveals the deep metabolic and vascular disruptions caused by chronic disease. The prevalence of ED among men with diabetes has been reported to range from 20% to more than 70%, depending on population characteristics and diagnostic criteria. Beyond being a sexual health concern, ED in diabetes signals endothelial dysfunction, impaired nitric oxide (NO) signaling, and often an accompanying deficiency of testosterone. Together, these elements create a perfect storm that undermines both quality of life and systemic health.
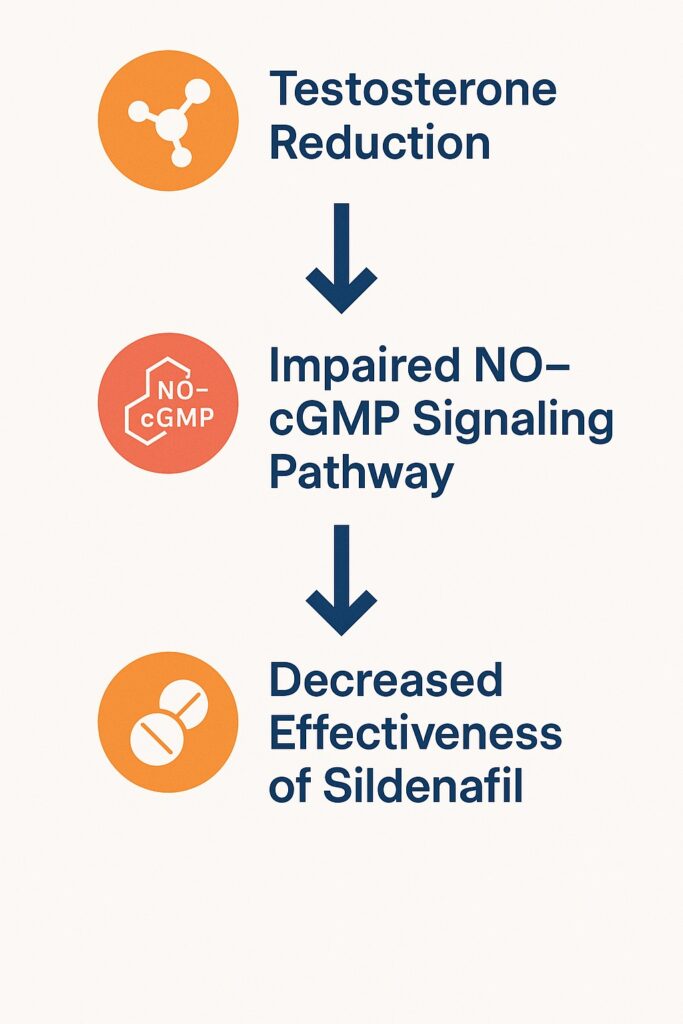
Testosterone deficiency, or hypotestosteronemia, is detected in up to half of men with T2DM. This hormonal deficit is associated with reduced libido, diminished erectile capacity, depression, insulin resistance, and osteoporosis. Meanwhile, phosphodiesterase-5 inhibitors (PDE5Is) such as sildenafil have become the cornerstone of ED therapy. They restore erectile function by enhancing the NO–cyclic guanosine monophosphate (cGMP) pathway. However, their efficacy varies significantly across patients, raising questions about whether underlying testosterone levels may influence the therapeutic outcome.
The study under review, conducted at Assalam Teaching Hospital in Mosul, Iraq, examined this very relationship. By correlating serum testosterone levels with erectile responses to sildenafil in diabetic men, it shed light on how endocrinology and pharmacology intersect in ED management. This article explores the background, methodology, findings, and implications of that research while situating it within the broader clinical landscape.
Erectile Dysfunction in Diabetes: The Complex Pathophysiology
Erectile function is an intricate physiological event that requires intact neurovascular, endocrine, and psychological components. Diabetes, unfortunately, impairs several of these pillars simultaneously.
Neuropathy, one of the classical complications of diabetes, disrupts the autonomic innervation of the corpora cavernosa. This diminishes the release of neurogenic nitric oxide (nNO), a key trigger of penile smooth muscle relaxation. Endothelial dysfunction, another hallmark of poorly controlled diabetes, undermines endotheliogenic NO (eNO) production, which is critical for maintaining erection once it begins. The result is both impaired initiation and reduced sustainability of penile rigidity.
Metabolic derangements compound the problem. Chronic hyperglycemia leads to the formation of advanced glycation end-products, which stiffen vascular tissues and impair signaling pathways. Dyslipidemia and hypertension further compromise penile arterial inflow. Testosterone deficiency adds an endocrine dimension: reduced libido, downregulated nNO synthase activity, and structural changes in erectile tissue. It is therefore unsurprising that ED prevalence in diabetic men is several times higher than in non-diabetic populations.
ED is not only a quality-of-life issue but also a cardiovascular risk marker. Its onset often precedes coronary artery disease by several years, making it a clinical red flag for underlying vascular pathology. For clinicians, this reinforces the importance of evaluating ED not merely as a urological symptom but as a systemic warning sign.
Testosterone: The Endocrine Driver of Sexual Function
Testosterone, secreted primarily by the Leydig cells of the testes, exerts far-reaching effects on male physiology. In the sexual domain, it regulates libido, penile tissue responsiveness, and spermatogenesis. While testosterone’s role in erectile capacity per se has been debated, experimental and clinical data indicate that deficiency downregulates NO synthase expression and weakens erectile tissue responsiveness.
In animal models, castration reduces erectile capacity, which can be restored with testosterone replacement. In humans, low testosterone is associated with decreased frequency and rigidity of erections. However, the threshold level at which hypogonadism translates into clinically significant ED is variable. Some men maintain adequate erectile capacity at relatively low testosterone levels, while others experience severe dysfunction at only modest declines.
In T2DM, the prevalence of hypotestosteronemia ranges from 30% to 50%. Mechanisms include obesity-related aromatization of testosterone to estradiol, insulin resistance impairing hypothalamic–pituitary signaling, and chronic inflammation suppressing Leydig cell function. Importantly, testosterone deficiency in this population is not only a sexual health issue but also correlates with worsened glycemic control, higher cardiovascular risk, and increased mortality.
Sildenafil and the NO–cGMP Pathway
Sildenafil citrate, approved by the FDA in 1998, revolutionized ED treatment. It selectively inhibits PDE5, the enzyme responsible for degrading cGMP in the corpus cavernosum. Under sexual stimulation, NO is released from nerve terminals and endothelial cells, activating guanylyl cyclase, which converts GTP into cGMP. Elevated cGMP lowers intracellular calcium, relaxes smooth muscle, and increases blood inflow into the penile sinusoids. The result: erection.
However, sildenafil’s pharmacological activity is contingent on intact NO signaling. Without adequate NO production, cGMP levels may never rise sufficiently for sildenafil to sustain them. This dependency explains why diabetic men with endothelial dysfunction, neuropathy, or hypotestosteronemia respond variably to PDE5Is. In essence, sildenafil enhances a pathway that must first be triggered; if the trigger is weak, the amplification may be inadequate.
Previous studies in non-diabetic hypogonadal men demonstrated that testosterone supplementation enhances sildenafil efficacy. Conversely, in men with normal testosterone, the correlation is less clear. The Iraqi study sought to determine whether similar dynamics operate in diabetic men with ED.
The Mosul Study: Design and Methods
Between 2009 and 2011, researchers in Mosul conducted a prospective comparative study at the Diabetic Clinic of Assalam Teaching Hospital. They enrolled 120 men with type 2 diabetes, aged 35–60, all of whom reported ED and/or diminished libido. Exclusion criteria included smoking, renal failure, major cardiovascular disease, medications known to impair sexual function, and ED predating diabetes.
Key data collected included patient age, diabetes duration, duration and severity of ED, libido changes, and drug history. Erectile function was assessed using simplified criteria derived from the International Index of Erectile Function (IIEF-5), focusing on erection strength and sexual desire. Serum total testosterone (TT) was measured using an enzyme-linked immunoassay. Hypotestosteronemia was defined as TT ≤10 nmol/L.
Participants were offered a therapeutic trial of sildenafil (50 mg, taken one hour before intercourse). They were counseled on side effects and instructed to use at least three doses before reporting outcomes. Responses were graded as percentage improvement in erection quality.
Statistical analyses included Student’s t-test, chi-square test, and Pearson correlation. A p-value of <0.05 was deemed significant.
Key Findings
The study revealed several notable outcomes:
- Prevalence of low testosterone: 36% (44 of 120) of diabetic men with ED had hypotestosteronemia, with mean TT levels of 7.6 nmol/L. The remainder had normal testosterone, averaging 16.5 nmol/L.
- Baseline comparisons: No significant differences in age, duration of diabetes, or severity of ED were observed between the low- and normal-testosterone groups.
- Response to sildenafil: Among hypotestosteronemic men, 80% reported improvement, with an average erection improvement of 43.7%. Among normotestosteronemic men, 87% improved, with a nearly identical average improvement of 44.7%.
- Testosterone correlation: Crucially, the degree of improvement in the hypotestosteronemic group was significantly correlated with testosterone levels. In other words, the lower the testosterone, the weaker the sildenafil effect. In the normotestosteronemic group, no such correlation existed.
These findings suggest that while both groups benefited from sildenafil, testosterone levels modulated the degree of improvement only among those with low baseline testosterone.
Clinical Interpretation
The study underscores several important points for clinicians. First, not all ED in diabetic men is driven by low testosterone; in fact, two-thirds of the cohort had normal TT levels. Second, sildenafil remains effective across both groups, but its potency is partly constrained by testosterone availability in hypotestosteronemic men. This interaction suggests a synergistic relationship between testosterone and PDE5Is: testosterone primes erectile tissue for NO responsiveness, and sildenafil sustains the NO–cGMP signal.
From a therapeutic standpoint, diabetic men with ED and low testosterone may benefit from combined therapy. Testosterone supplementation could optimize baseline androgen status, thereby enhancing sildenafil’s efficacy. This approach aligns with other interventional studies showing improved outcomes with dual therapy in hypogonadal men.
Another clinically relevant observation is that hypotestosteronemia in diabetes does not correlate strongly with age or diabetes duration. This suggests that poor glycemic control and metabolic factors may be more decisive than chronological age. Consequently, routine testosterone testing in diabetic men with ED is justified regardless of patient age.
Broader Implications: Sexual Health as a Metabolic Marker
Sexual dysfunction in diabetes is not an isolated event—it is a systemic signal. Testosterone deficiency is associated with increased cardiovascular risk, insulin resistance, and adverse body composition. ED itself is a sentinel marker of endothelial dysfunction. Therefore, assessing sexual health provides clinicians with an opportunity to detect broader systemic disease and intervene early.
The Mosul study strengthens the case for integrated management. Rather than treating ED as an isolated complaint, endocrinological evaluation should be routine. Identifying hypotestosteronemia enables personalized therapy: testosterone replacement when indicated, combined with PDE5Is for optimal effect. This integrated approach not only restores sexual function but may also improve metabolic outcomes.
Limitations and Research Gaps
While insightful, the study had limitations. Its relatively small sample size and single-center design limit generalizability. Testosterone was measured only once, and diurnal variations were not accounted for. Free testosterone, a more precise marker of androgen status, was not evaluated. Additionally, the study did not assess long-term outcomes of combined therapy or explore other determinants of ED, such as psychological factors or comorbidities like hypertension.
Future research should address these gaps through multicenter randomized controlled trials, incorporating larger cohorts, longer follow-up, and comprehensive hormonal profiling. Studies should also explore whether testosterone optimization improves not only erectile outcomes but also glycemic control and cardiovascular endpoints.
Conclusion
The interplay between testosterone, diabetes, and erectile dysfunction is complex but clinically significant. This study demonstrated that while sildenafil is effective in diabetic men with ED regardless of testosterone status, the degree of improvement in hypotestosteronemic men is directly proportional to serum testosterone levels. The findings support the rationale for screening testosterone in diabetic men presenting with ED and considering combined testosterone and PDE5I therapy when deficiency is identified.
As diabetes prevalence continues to rise globally, addressing its complications—including ED—requires nuanced, multidisciplinary strategies. By linking endocrinology and sexual medicine, clinicians can offer treatments that restore not just erections but also dignity, confidence, and systemic health.
FAQ
1. Should all diabetic men with erectile dysfunction have their testosterone tested?
Yes. Given the high prevalence of hypotestosteronemia in this population, routine testing helps guide therapy. Identifying low testosterone allows clinicians to consider supplementation, which may improve both erectile outcomes and metabolic health.
2. Can sildenafil work if testosterone levels are very low?
Sildenafil can still induce improvement, but its effectiveness may be blunted in men with marked testosterone deficiency. In such cases, raising testosterone levels may enhance responsiveness to the drug.
3. Is combined therapy with testosterone and sildenafil safe and effective?
Clinical studies suggest that combination therapy is safe for most men and can provide superior outcomes compared to either treatment alone, particularly in hypogonadal patients. However, therapy should always be individualized and supervised by a healthcare professional.