Introduction
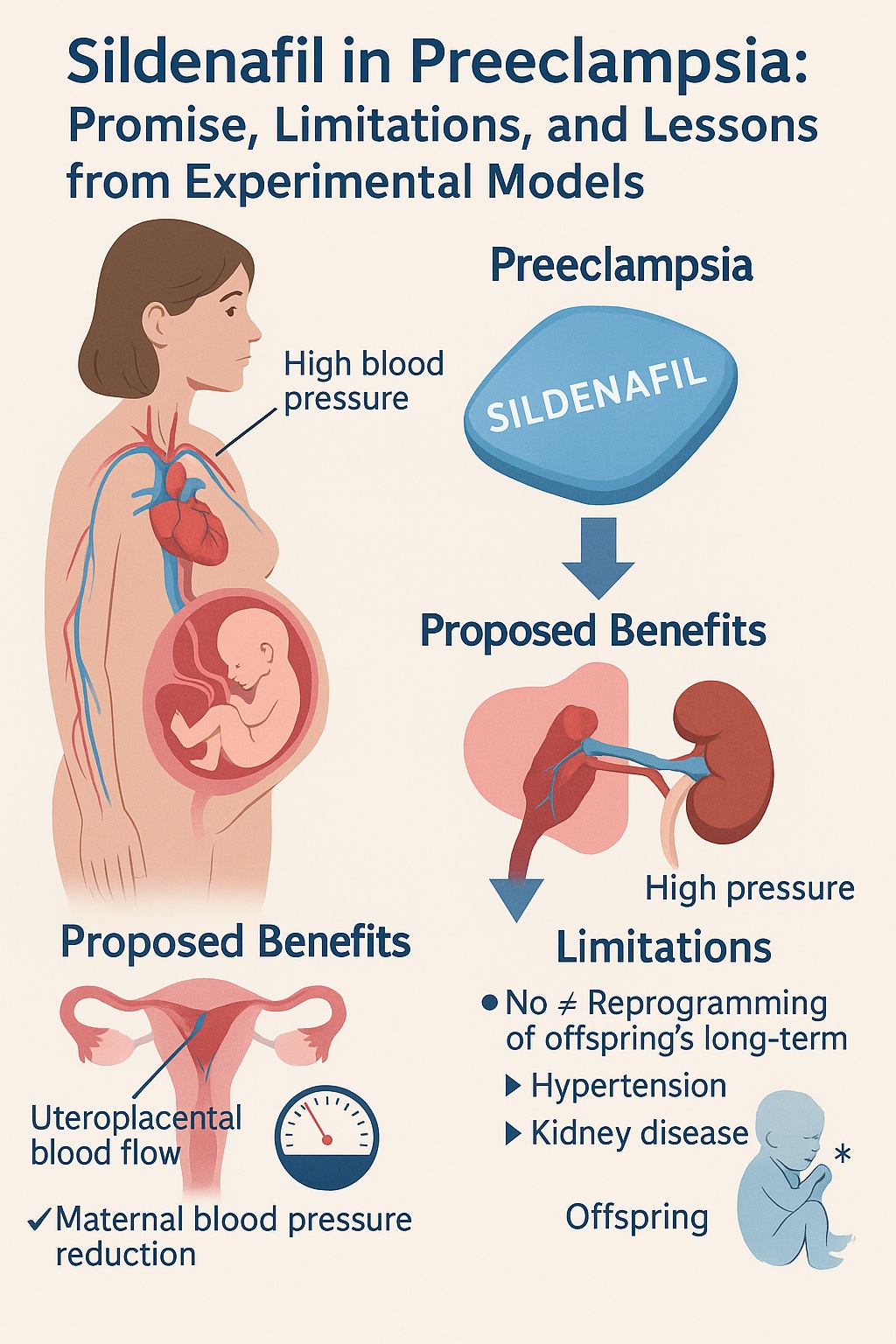
Preeclampsia remains one of the most formidable complications of pregnancy, affecting up to 8% of expectant mothers worldwide. Characterized by new-onset hypertension, proteinuria, and a spectrum of systemic disturbances, it continues to challenge obstetricians and researchers alike. Historically regarded as a disorder that resolves with delivery, modern science has revealed a much more troubling reality: preeclampsia is not confined to pregnancy. Instead, it casts a long shadow across the lives of both mothers and their offspring, leaving behind increased risks of cardiovascular and renal disease that manifest decades later.
Among the many candidate therapies investigated to mitigate preeclampsia, sildenafil citrate — more widely recognized for its role in treating erectile dysfunction and pulmonary hypertension — has emerged as a particularly intriguing option. Sildenafil’s pharmacological mechanism, involving inhibition of phosphodiesterase-5 (PDE-5) and potentiation of nitric oxide (NO)-mediated vasorelaxation, makes it a natural candidate for addressing the endothelial dysfunction central to preeclampsia. Early studies suggested maternal benefits and even improvements in fetal growth. The tantalizing hypothesis followed: could intrapartum sildenafil not only ease maternal disease but also “reprogram” the developmental trajectory of exposed offspring, sparing them from future hypertension and kidney disease?
A recent study in the Dahl SS/Jr rat model sought to answer precisely this question. By carefully examining the blood pressure, renal function, and stress responses of offspring from sildenafil-treated and untreated preeclamptic pregnancies, the investigators probed the intergenerational ripple effects of maternal therapy. The findings, while nuanced, tell a sobering story that tempers early enthusiasm.
In this article, we will explore the scientific rationale, experimental design, findings, and clinical implications of this research. Along the way, we will situate sildenafil within the broader landscape of preeclampsia management, critically appraise the evidence, and reflect on what lessons it offers for the future of obstetric and cardiovascular medicine.
The Burden of Preeclampsia Beyond Pregnancy
Preeclampsia is far more than an obstetric inconvenience. For mothers, it increases the likelihood of chronic hypertension, cardiovascular disease, and renal dysfunction in later life. For offspring, the consequences are equally concerning. Epidemiological studies consistently show that children born to preeclamptic pregnancies exhibit higher blood pressure, increased risk of stroke, and even altered vascular reactivity as they mature. This transgenerational link is a compelling example of the Developmental Origins of Health and Disease (DOHaD) theory, which proposes that adverse intrauterine environments leave lasting epigenetic and physiological imprints.
The economic burden of preeclampsia mirrors its medical impact. Health care costs for mothers and infants affected by the condition are several times higher than those of uncomplicated pregnancies. This dual impact — clinical and economic — has intensified the urgency of discovering effective therapies that extend beyond maternal stabilization and early delivery.
Despite decades of research, therapeutic options remain disappointingly limited. The current standard of care emphasizes close monitoring, cautious use of antihypertensive medications, seizure prophylaxis with magnesium sulfate, and timely delivery. These measures may save lives, but they do not alter the underlying endothelial dysfunction, nor do they modify long-term risks for mother or child. Against this backdrop, experimental interventions such as antioxidants, statins, angiogenic modulators, and vasodilators like sildenafil have attracted considerable attention.
Why Sildenafil?
The rationale for sildenafil in preeclampsia rests on a straightforward chain of reasoning. Preeclampsia is associated with reduced bioavailability of nitric oxide, impaired vasodilation, and heightened systemic vascular resistance. Sildenafil, by inhibiting PDE-5, prevents degradation of cyclic guanosine monophosphate (cGMP), thereby enhancing the downstream effects of NO and promoting vascular relaxation.
This pharmacological action is not merely theoretical. PDE-5 is expressed in the uterine vasculature, and sildenafil has been shown to improve relaxation of myometrial vessels. In animal models, maternal sildenafil improved uteroplacental perfusion, prolonged gestation, reduced maternal blood pressure, and increased litter size. Human studies, though smaller and less definitive, reported reduced uteroplacental resistance and modest reductions in maternal mean arterial pressure. There was also evidence of improved fetal growth, lending credibility to the notion that sildenafil might mitigate intrauterine growth restriction associated with preeclampsia.
With these observations in mind, researchers advanced a bold hypothesis: could maternal sildenafil not only treat the immediate hemodynamic consequences of preeclampsia but also permanently reshape the developmental programming of offspring, insulating them from future cardiovascular and renal disease? If true, such a discovery would represent a paradigm shift in both obstetric and preventive medicine.
The Experimental Model: Dahl SS/Jr Rats
The study in question employed the Dahl salt-sensitive rat, a model known for its spontaneous development of hypertension and superimposed preeclampsia when subjected to a high-salt diet. This model captures key features of the human condition, including maternal hypertension, proteinuria, and fetal growth restriction. Importantly, it also provides a controlled environment to examine offspring outcomes in a way that human studies cannot ethically achieve.
In the experiment, pregnant dams were randomized at gestational day 10 to receive either standard diet or diet supplemented with sildenafil citrate at a dose of 50 mg/kg/day. Treatment continued until delivery. Offspring were weaned at 4 weeks and allowed to age on standard diet until 3 months. At that stage, they were further divided into three experimental conditions: baseline (no intervention), high-salt diet (2% NaCl for 4 weeks), or low-dose angiotensin II infusion (200 ng/kg/min for 2 weeks). This design permitted evaluation of both baseline physiology and responses to secondary stressors, reflecting real-world challenges such as dietary salt excess and neurohormonal activation.
Throughout the study, investigators measured blood pressure, renal function, proteinuria, and markers of renal injury. Gene expression analyses provided molecular insight, and careful statistical methods ensured rigor.
Key Findings
The results of this carefully executed study were both illuminating and sobering. At baseline, offspring of sildenafil-treated and untreated dams displayed no significant differences in systolic blood pressure, proteinuria, nephrin excretion, or creatinine clearance. Both groups experienced the expected rise in blood pressure and proteinuria with age, particularly in male offspring, but sildenafil exposure during gestation did not confer lasting protection.
When exposed to a high-salt diet, male offspring of sildenafil-treated dams did demonstrate an attenuated rise in blood pressure compared to controls. This sex-specific effect suggested a modest protective influence of maternal therapy on salt-sensitive hypertension. However, this benefit was not observed in female offspring, nor did it translate into measurable improvements in renal injury markers.
When challenged with angiotensin II infusion, offspring of sildenafil-treated dams fared no better than controls. Blood pressure increased across all groups, and no differences in renal function or injury were observed. This lack of effect underscored the limits of sildenafil’s protective potential.
Molecular analyses further supported these conclusions. Expression of genes involved in renal injury, oxidative stress, and inflammation did not differ significantly between treatment groups, with only scattered and modest variations that lacked consistent functional correlates.
The overarching conclusion was clear: maternal sildenafil treatment does not reprogram the risk of hypertension or chronic kidney disease in offspring of preeclamptic pregnancies. Any benefits are modest, sex-specific, and limited to salt-sensitive blood pressure responses in males.
Interpreting the Results
These findings challenge the appealing hypothesis that a simple pharmacological intervention during pregnancy could permanently alter offspring health trajectories. Several interpretations are possible.
First, it may be that sildenafil’s effects are too localized or transient to exert lasting developmental reprogramming. While it improves fetoplacental perfusion and maternal hemodynamics in the short term, these changes may not translate into the epigenetic modifications required for long-term benefit.
Second, the pathogenesis of offspring hypertension and renal disease after preeclampsia is likely multifactorial. Beyond impaired NO signaling, factors such as antiangiogenic proteins (sFlt-1, soluble endoglin), oxidative stress, and genetic predispositions play roles. Targeting a single pathway with sildenafil may therefore be insufficient to counteract the broader programming insults.
Third, sex-specific effects complicate interpretation. Male offspring appeared to gain modest protection against salt-induced hypertension, while females did not. Whether this reflects hormonal influences, differences in renal handling of sodium, or other unknown factors remains uncertain. Such sex-specific programming effects are well recognized in developmental biology, reminding us that “one-size-fits-all” solutions are rarely applicable.
Finally, methodological limitations deserve mention. Animal studies, while invaluable, cannot fully replicate human physiology or the complexity of preeclampsia. Moreover, the interventions tested may have elicited maximal responses, obscuring subtler effects. Nonetheless, the study’s rigorous design lends credibility to its conclusions.
Clinical Implications
For clinicians and researchers, the message is both sobering and instructive. Sildenafil is unlikely to serve as a “developmental reprogrammer” that rescues offspring of preeclamptic pregnancies from long-term cardiovascular and renal risk. Its role, if any, may be confined to maternal symptom relief and modest improvements in fetal growth or short-term outcomes.
This does not diminish the importance of continuing to explore pharmacological strategies for preeclampsia. Rather, it emphasizes the need for multifaceted approaches that address endothelial dysfunction, oxidative stress, angiogenic imbalance, and immune dysregulation simultaneously. It also underscores the importance of long-term follow-up in both animal models and human trials, as short-term benefits may not equate to lifelong protection.
In practical terms, clinicians should remain cautious. While sildenafil may hold value in carefully selected scenarios, it cannot yet be recommended as a routine therapy for preeclampsia, let alone as a means of safeguarding offspring health. Ongoing research must clarify its safety, efficacy, and long-term impact.
Future Directions
The quest to improve outcomes in preeclampsia is far from over. Future research must grapple with several key challenges:
- Understanding mechanisms: Detailed exploration of epigenetic changes, vascular development, and hormonal influences is needed to unravel how preeclampsia imprints long-term risk on offspring.
- Multimodal therapies: Combination approaches that target multiple pathogenic pathways may prove more effective than single agents.
- Personalized medicine: Recognizing sex-specific and genetic differences will be essential to tailoring interventions.
- Long-term follow-up: Both preclinical and clinical studies must extend beyond delivery and early infancy to capture the enduring consequences of interventions.
In the meantime, preventive strategies such as low-dose aspirin, optimization of maternal health before pregnancy, and vigilant monitoring remain the bedrock of clinical care.
Conclusion
The allure of a single pharmacological agent capable of rewriting the developmental script for offspring of preeclamptic pregnancies is undeniable. Sildenafil, with its elegant mechanism and early promise, seemed a leading candidate. Yet the latest evidence reminds us that biology rarely yields to simple solutions. While sildenafil may improve maternal hemodynamics and modestly temper salt-sensitive hypertension in male offspring, it does not fundamentally alter the long-term risks of hypertension or chronic kidney disease.
As is so often the case in medicine, the lesson is one of humility. Progress against preeclampsia will come not from a silver bullet but from sustained, multifaceted efforts that integrate clinical care, mechanistic science, and long-term vigilance. In the meantime, the shadows of preeclampsia continue to remind us of the profound and enduring influence of the intrauterine environment on lifelong health.
FAQ
1. Can sildenafil be safely used to treat preeclampsia in pregnant women?
Evidence suggests that sildenafil may modestly improve uteroplacental blood flow and maternal blood pressure in some cases, but its routine use remains experimental. Safety concerns, particularly regarding offspring outcomes, mean it is not currently recommended as standard therapy.
2. Does preeclampsia always increase the child’s risk of hypertension later in life?
Not always, but the risk is significantly higher compared to children from uncomplicated pregnancies. Factors such as severity of maternal disease, gestational age at delivery, and genetic predispositions influence individual outcomes.
3. What strategies currently exist to prevent or reduce the risk of preeclampsia?
Low-dose aspirin is widely recommended for high-risk women. Other approaches include controlling maternal hypertension, optimizing weight and metabolic health before pregnancy, and close monitoring during gestation. Experimental therapies, including sildenafil, remain under investigation but are not yet standard of care.