Introduction: A Drug of Paradoxical Promise
Sildenafil citrate, more famously known as the little blue pill that revolutionized the treatment of erectile dysfunction, has had an unexpectedly adventurous career in medicine. Initially developed as a vasodilator for angina, it found global fame for its “side effect.” Yet, its pharmacological core — the inhibition of phosphodiesterase type 5 (PDE5) — endows it with far broader physiological implications than anyone could have predicted in the 1980s.
By enhancing cyclic guanosine monophosphate (cGMP) and potentiating nitric oxide (NO)-mediated vasodilation, sildenafil improves blood flow in a selective and tissue-specific manner. This vasorelaxant and anti-remodeling effect soon made it a candidate for conditions ranging from pulmonary hypertension to Raynaud’s disease.
Then, in a twist few anticipated, sildenafil entered obstetrics. Researchers proposed that its endothelial-modulating properties could restore uteroplacental perfusion, alleviate fetal growth restriction, and perhaps even save fetuses otherwise doomed by placental insufficiency. Over the past two decades, the drug has been studied in virtually every obstetric complication involving abnormal vascular tone — from preeclampsia and fetal growth restriction (FGR) to congenital diaphragmatic hernia (CDH).
But as the story unfolded, the promise became intertwined with controversy. What began as a tale of translational ingenuity evolved into one of scientific caution — a reminder that in medicine, not every plausible idea translates safely from bench to bedside.
The Biological Rationale: Revisiting the Nitric Oxide Pathway
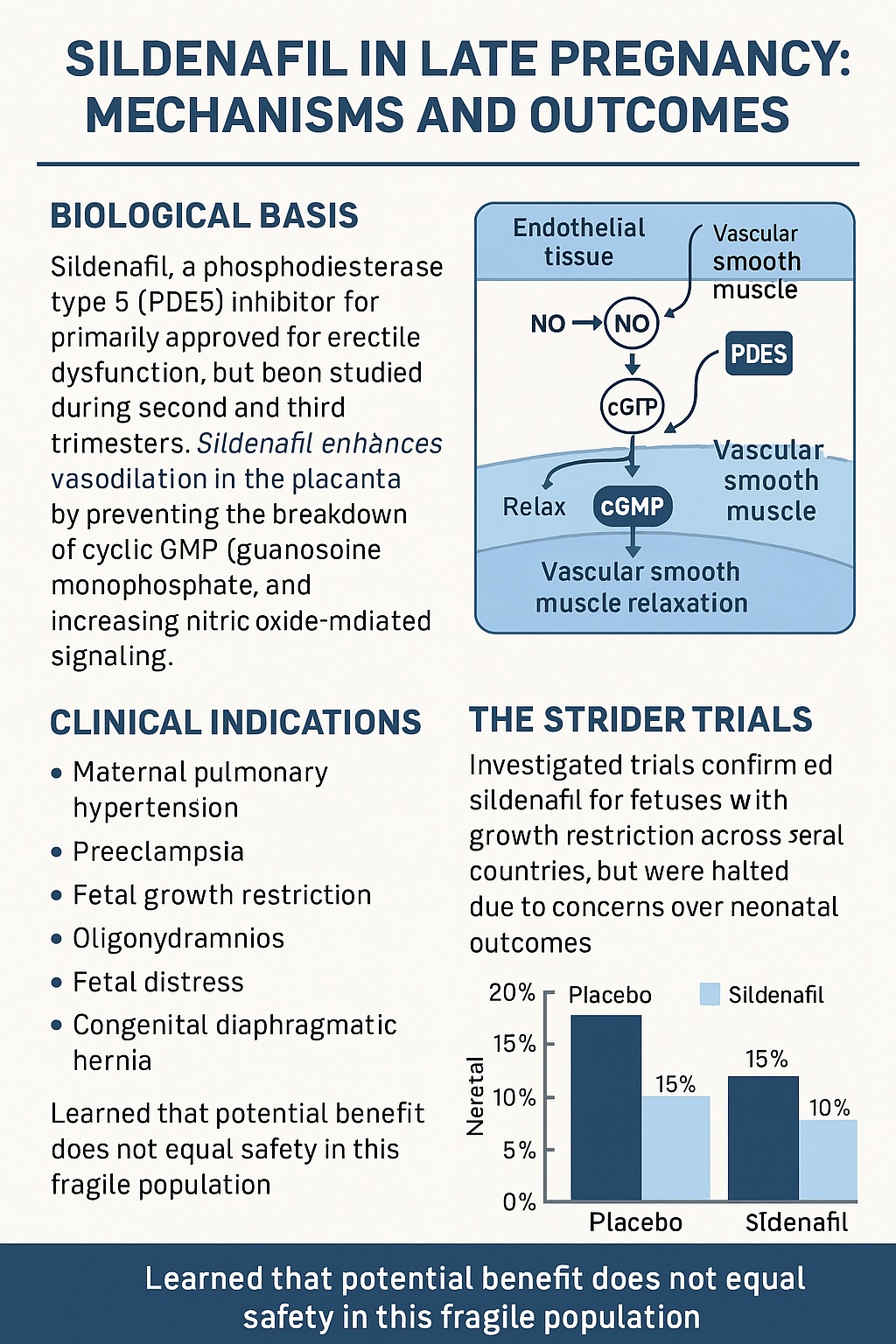
The conceptual appeal of sildenafil in pregnancy is elegant. During normal gestation, the nitric oxide–cGMP pathway orchestrates maternal vascular adaptation, promoting low-resistance uteroplacental circulation essential for fetal growth. Endothelial nitric oxide synthase (eNOS) generates NO, which activates guanylate cyclase, increasing cGMP within vascular smooth muscle cells. This cascade causes relaxation, improved perfusion, and inhibition of smooth muscle proliferation.
PDE5, abundant in pulmonary arteries and uterine vasculature, degrades cGMP. By blocking PDE5, sildenafil enhances the half-life of cGMP and thereby amplifies vasodilation. In theory, this mechanism could correct endothelial dysfunction and reverse vasoconstrictive remodeling, both hallmarks of several pregnancy disorders.
Unlike systemic vasodilators, sildenafil’s hemodynamic effect is relatively localized, producing only modest decreases in systemic blood pressure — an advantage in pregnancy, where hypotension can harm the fetus. Its metabolism via hepatic CYP3A4 and CYP2C19 enzymes, which are active even in utero, further supports its feasibility for antenatal use.
This biologic reasoning inspired a surge of interest across multiple obstetric domains, with each indication offering a unique combination of promise and peril.
Sildenafil and Maternal Pulmonary Hypertension: From Risk to Relief
Pregnancy in the setting of pulmonary arterial hypertension (PAH) remains one of the most perilous clinical scenarios in obstetrics. The physiological volume expansion and increased cardiac output can precipitate right ventricular failure, leading to catastrophic maternal and fetal outcomes.
Conventional PAH therapies are limited during pregnancy due to teratogenicity or systemic toxicity. Sildenafil, already approved for PAH in non-pregnant adults, emerged as a relatively safe and rational alternative. Case reports and small series from Europe and North America documented successful pregnancies in women maintained on sildenafil throughout gestation, with improved pulmonary pressures and no clear fetal harm.
These findings positioned sildenafil as a lifesaving adjunct in select, high-risk PAH cases. Although randomized trials are lacking, the consistency of anecdotal evidence supports cautious continuation of sildenafil in women who become pregnant while on therapy — a rare example where its off-label obstetric use remains clinically justified.
Preeclampsia: Targeting Endothelial Dysfunction
Preeclampsia — that enigmatic hypertensive disorder of pregnancy — is perhaps the most compelling context in which sildenafil was explored. Characterized by abnormal placentation, antiangiogenic factor release, and systemic endothelial dysfunction, it has long been viewed as a disease of failed vascular adaptation.
The idea was simple: if preeclampsia is a state of nitric oxide deficiency and vasoconstriction, why not pharmacologically boost the NO–cGMP pathway? Sildenafil, as a PDE5 inhibitor, could in theory restore vascular balance, reduce uterine artery resistance, and prolong gestation.
Animal studies offered encouraging data — reductions in blood pressure, improved renal function, and increased fetal weights in preeclamptic rat models. However, translation to human pregnancy proved elusive. Two randomized controlled trials, though small, offered mixed results. One showed reduced maternal blood pressure without prolonging pregnancy; the other found a modest four-day increase in gestational age at delivery. Neither demonstrated improvements in perinatal survival.
Despite its physiologic appeal, sildenafil did not become a therapeutic mainstay for preeclampsia. The evidence suggested transient hemodynamic benefit without meaningful obstetric gain. In the delicate balance of maternal-fetal safety, “well-tolerated but ineffective” is rarely sufficient justification for routine use.
Preterm Labor: Relaxation Without Results
The search for an ideal tocolytic agent — one that safely halts preterm labor — has been a perpetual quest. Sildenafil, with its smooth muscle relaxant properties, seemed an intriguing candidate.
In vitro, sildenafil suppressed myometrial contractility in rat and human models through NO-dependent pathways. Yet, the concentrations required to achieve relaxation were pharmacologically unrealistic, risking systemic hypotension. One small Iranian trial suggested that vaginal sildenafil, combined with nifedipine, prolonged latency and improved neonatal respiratory outcomes. However, the data were sparse, safety outcomes underreported, and subsequent studies failed to replicate these effects.
Thus, while sildenafil can relax uterine smooth muscle in a Petri dish, clinical translation faltered. The uterus, it turns out, is a more complex organ than the corpus cavernosum.
Fetal Growth Restriction: From Hope to Halted Trials
Among all pregnancy disorders studied, fetal growth restriction (FGR) generated the greatest excitement — and ultimately, the greatest disappointment — for antenatal sildenafil.
The logic was persuasive. In FGR, poor placental perfusion leads to hypoxia and nutrient deprivation. By dilating uteroplacental vessels, sildenafil could enhance fetal oxygenation and growth. Early pilot studies from Canada and Iran showed promising Doppler changes and improved growth velocity. Enthusiasm grew, culminating in the formation of the multinational STRIDER consortium, which sought to rigorously evaluate sildenafil across multiple continents.
Then came the reckoning. The UK and Australasian STRIDER trials reported no benefit in survival or birthweight. More concerningly, the Dutch STRIDER trial was terminated early after interim analyses suggested a higher incidence of neonatal pulmonary hypertension and mortality in sildenafil-exposed infants. Though causality remains debated, the ethical weight of those findings was decisive.
The once-hopeful narrative of sildenafil as a “placental perfusion therapy” was abruptly halted. The medical community was reminded that vasodilation in the fetus is not always synonymous with benefit. In some contexts, it may disrupt adaptive vascular mechanisms such as “brain-sparing,” leading to unintended harm.
Oligohydramnios and Fetal Distress: Early Sparks, Limited Substance
Sildenafil’s use in oligohydramnios (low amniotic fluid) was based on a tenuous hypothesis — that enhanced uteroplacental perfusion could increase fetal renal blood flow and thereby boost urine production. A small randomized trial initially suggested benefit but was later retracted due to data fabrication. No credible evidence currently supports this indication.
More promising, however, was the RIDSTRESS trial, which explored sildenafil as a means to prevent intrapartum fetal distress. Conducted in Australia, the study demonstrated a 51% reduction in emergency operative deliveries and fewer pathological fetal heart rate patterns. Importantly, no adverse maternal or neonatal outcomes were reported. While these findings are preliminary and the study was underpowered for neonatal outcomes, they offer a rare glimmer of positive potential — one that warrants larger phase III validation.
Congenital Diaphragmatic Hernia: Treating the Fetus Before Birth
If sildenafil’s role in obstetric vascular disorders has been fraught, its potential in fetal structural anomalies remains compelling. Congenital diaphragmatic hernia (CDH), a condition of pulmonary hypoplasia and vascular remodeling, represents a devastating pathology where prenatal intervention could alter lifelong outcomes.
Experimental models in rats, rabbits, and lambs consistently demonstrated that maternal sildenafil reduced pulmonary artery wall thickness, increased vessel density, and improved postnatal gas exchange. The drug appeared to promote normal pulmonary vascular development, counteracting the maladaptive remodeling central to CDH pathology.
These findings inspired a phase I/IIb clinical trial assessing the pharmacokinetics and safety of antenatal sildenafil in CDH pregnancies. Yet, the shadow of the STRIDER controversy halted progress; regulators suspended recruitment over safety concerns extrapolated from unrelated indications.
Still, many researchers argue that the mechanistic rationale for CDH differs fundamentally from FGR, and the potential for benefit — particularly when combined with fetal tracheal occlusion therapy — remains strong. The story here is not closed but merely paused.
Safety, Tolerability, and the Ethics of Innovation
Safety data across trials present a generally reassuring picture for maternal health. Reported side effects — headache, flushing, visual disturbance, dyspepsia — mirror those seen in non-pregnant users and are typically mild. Serious adverse events, including systemic hypotension, were rare.
The fetal story, however, is more nuanced. Apart from the Dutch STRIDER findings, most studies observed no consistent pattern of teratogenicity or increased neonatal morbidity. Still, the possibility of drug-induced pulmonary maladaptation in neonates underscores the need for caution when manipulating vascular tone during late gestation.
The ethical lessons are equally vital. The rapid translation of sildenafil from animal models to human pregnancy, driven by understandable urgency to save compromised fetuses, bypassed parts of the translational pipeline. The field now recognizes the necessity of rigorous preclinical modeling and mechanistic clarity before embarking on large-scale human trials in such vulnerable populations.
Lessons from the STRIDER Saga: Science, Media, and Medicine
Few obstetric trials have captured public attention as dramatically as STRIDER. The decision to halt the Dutch arm was accompanied by intense media coverage, sometimes sensationalizing the story as “the deadly Viagra trial.” This conflation of scientific uncertainty with public panic damaged both the research community and the perception of fetal therapy as a field.
Yet, the episode provided an opportunity for reflection. Researchers re-examined preclinical data, identified weaknesses in animal models, and debated whether species differences in placental transfer and vascular reactivity might explain the divergent outcomes. It also prompted stronger ethical frameworks for maternal-fetal drug trials, emphasizing independent monitoring and transparent communication.
Perhaps the greatest legacy of STRIDER is a humbling reminder that biological plausibility is not proof of benefit. Even the most rational therapeutic hypothesis can unravel when exposed to the complexity of human physiology.
Looking Forward: Where Sildenafil Still Belongs
Despite the turbulence, sildenafil remains an invaluable pharmacologic tool in several maternal-fetal contexts. For maternal PAH, its role is justified, supported by mechanistic logic and case-based success. For fetal distress, early data encourage further exploration under strict ethical oversight. And for CDH, the mechanistic rationale continues to merit preclinical and cautious clinical study.
However, for preeclampsia, FGR, and oligohydramnios, the consensus is firm: the evidence does not support continued experimental or off-label use. The scientific community has, appropriately, shifted from unbridled optimism to measured realism.
The broader implication extends beyond sildenafil itself. The story underscores how fetal medicine — at the intersection of pharmacology, ethics, and innovation — must balance desperation with discipline. In pursuing cures for unborn patients, one must resist the temptation to let hope outpace evidence.
Conclusion: Trials, Tribulations, and the Maturing of Fetal Therapy
The saga of sildenafil in late pregnancy is both cautionary and inspiring. It began with the best of intentions: to repurpose a safe vasodilator for the benefit of mothers and fetuses suffering from vascular compromise. Along the way, it illuminated the challenges inherent in translating pharmacologic theory into perinatal practice.
Sildenafil is not the panacea for placental dysfunction many once envisioned. But its exploration has yielded invaluable insights — into maternal-fetal hemodynamics, into the ethics of experimental obstetrics, and into the fragile optimism that drives medical progress. In the end, it reminds us that even when a therapeutic journey ends in disappointment, the science gained along the way can still serve the future.
FAQ: Sildenafil Use in Late Pregnancy
1. Is sildenafil safe to use during pregnancy?
Current evidence suggests that sildenafil is generally safe for the mother but may pose risks to the fetus in certain contexts, particularly in fetal growth restriction. It should only be used in research settings or for maternal conditions like pulmonary hypertension under specialist supervision.
2. Why were the STRIDER trials stopped?
The Dutch STRIDER trial was halted after interim analysis indicated increased rates of neonatal pulmonary hypertension and mortality in the sildenafil group. This prompted a precautionary halt across all related studies.
3. Does sildenafil have any future in obstetric medicine?
Possibly. Its potential in maternal pulmonary hypertension and congenital diaphragmatic hernia remains promising, provided future research adheres to rigorous translational and ethical standards.