Introduction
Pulmonary arterial hypertension (PAH) has long been a therapeutic challenge. Defined by increased pulmonary vascular resistance leading to progressive right heart failure, it transforms the physiology of the cardiopulmonary system into a relentless spiral of dyspnea, hypoxemia, and ultimately premature death. Advances in pharmacology over the past three decades have provided patients with new hope, but mortality rates remain stubbornly high. Among the armamentarium of therapies, sildenafil citrate, a phosphodiesterase type 5 (PDE5) inhibitor, has emerged as a mainstay. Its vasodilatory effect, mediated through enhancement of nitric oxide–cGMP signaling, makes it particularly effective in reducing pulmonary arterial pressure and improving exercise capacity.
Since the pivotal SUPER-1 trial, sildenafil has been approved globally as a treatment for PAH, typically at a dose of 20 mg three times daily. Yet, questions lingered. Was this dose optimal? Could higher doses produce greater survival benefits, or would they only add toxicity? Conversely, might lower doses suffice for certain patients, reducing pill burden and side effects? These questions were not trivial. For chronic conditions like PAH, where patients may remain on therapy for years, dose optimization is as important as drug selection.
The 2024 multicenter randomized trial by Hoeper and colleagues sought to finally provide clarity. By comparing low, medium, and high doses of sildenafil in adults with PAH, the investigators asked the most fundamental of clinical questions: does dose influence survival? The results, though nuanced, carry profound implications for daily practice, regulatory standards, and our broader understanding of PAH management.
The Rationale Behind Dose Exploration
The decision to explore sildenafil dosing was driven by both clinical experience and regulatory precedent. While the initial licensing dose of 20 mg three times daily was chosen primarily for regulatory consistency rather than strict pharmacodynamic superiority, clinicians worldwide began experimenting with higher doses. Real-world registries indicated that many patients were receiving 40 mg or even 80 mg three times daily, sometimes with apparent benefit.
Mechanistically, the logic was straightforward: higher doses should lead to greater PDE5 inhibition, higher intracellular cGMP, and therefore more sustained vasodilation. However, biology is rarely linear. Pharmacokinetics suggested a plateau effect at higher concentrations, and safety concerns—such as hypotension, visual disturbances, and systemic side effects—raised caution. Furthermore, without a controlled mortality trial, it was impossible to know whether such practices were therapeutic bravado or genuine clinical advancement.
On the other end of the spectrum, some clinicians hypothesized that lower doses might suffice in stable patients, reducing cost and side effects without compromising efficacy. For health systems and patients alike, the economic stakes were not negligible. Thus, the trial addressed both ends of the spectrum: could we justify higher doses, and could we safely economize with lower ones?
Study Design: A Pragmatic Multicenter Effort
The investigators designed a randomized, double-blind, multicenter trial enrolling several hundred patients with confirmed PAH across multiple international sites. Patients included in the study had WHO Group 1 PAH, encompassing idiopathic, heritable, drug-induced, and connective tissue disease–associated forms. Importantly, participants were either treatment-naïve or stabilized on background therapy such as endothelin receptor antagonists.
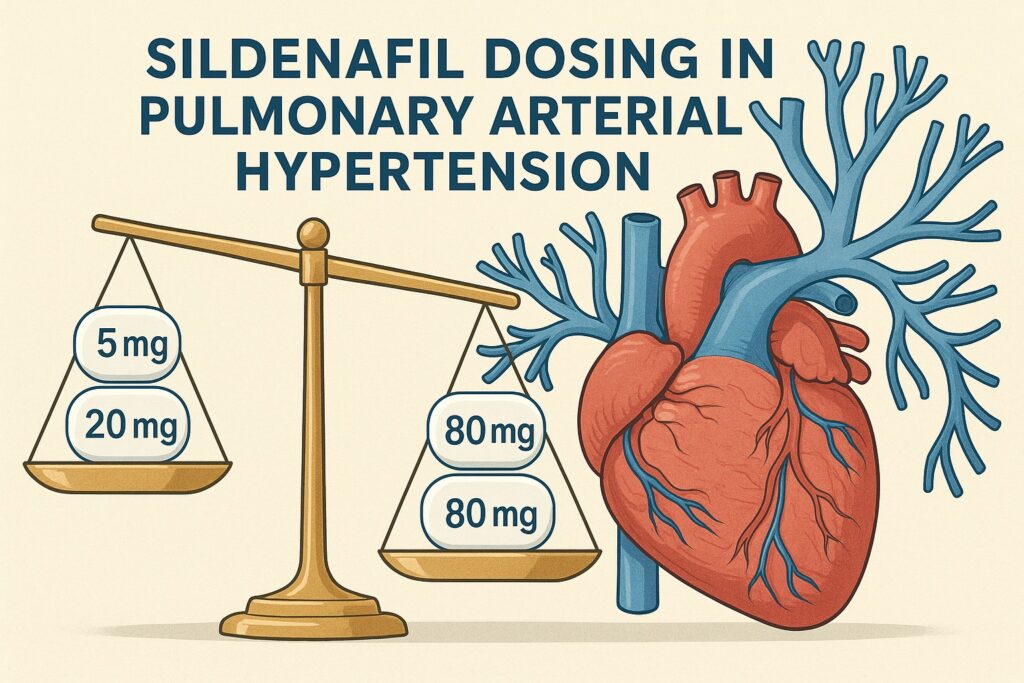
Patients were randomized to one of three arms:
- Low dose: 5 mg three times daily.
- Standard dose: 20 mg three times daily (the regulatory reference).
- High dose: 80 mg three times daily.
The primary endpoint was all-cause mortality at 16 weeks, with extended follow-up for survival curves. Secondary endpoints included six-minute walk distance (6MWD), WHO functional class, biomarkers such as NT-proBNP, and safety/tolerability measures. The trial was rigorously monitored, with central adjudication of outcomes and independent safety boards ensuring integrity.
By including mortality as the primary endpoint, the investigators raised the bar. Many PAH studies rely on surrogate markers such as exercise capacity or time to clinical worsening. Mortality, however, speaks to the ultimate goal of therapy: prolonging life while preserving function.
Mortality Outcomes: Dose Matters, but Not as Expected
The results were striking. At 16 weeks, mortality rates differed significantly across groups. The low-dose arm demonstrated a markedly higher death rate compared to both the standard and high-dose arms. Patients receiving only 5 mg three times daily were inadequately protected, confirming that subtherapeutic dosing in PAH can have devastating consequences.
In contrast, the high-dose arm (80 mg three times daily) did not outperform the standard 20 mg dose in terms of survival. Mortality rates were statistically indistinguishable between these two groups. In other words, higher doses did not confer additional protection. The survival benefit plateaued at the standard 20 mg regimen.
These findings underscore a critical lesson in clinical pharmacology: more is not always better. Sildenafil’s effect on the pulmonary vasculature appears to saturate at standard dosing, with little to gain and potentially more to lose by escalating further. Conversely, lowering the dose proved dangerous, leading to excess mortality.
The trial therefore reaffirmed the regulatory standard of 20 mg three times daily as the optimal dose for survival benefit. Real-world deviations toward higher or lower regimens should now be reconsidered in light of these definitive data.
Functional and Biomarker Outcomes
Beyond survival, the trial provided valuable insights into secondary measures of disease burden.
Six-minute walk distance (6MWD), the traditional workhorse of PAH trials, improved significantly in both the standard and high-dose arms, with no meaningful difference between them. The low-dose group, unsurprisingly, demonstrated minimal gains. This reinforces that the standard dose achieves maximal functional benefit.
WHO functional class improved in a substantial proportion of patients in both standard and high-dose arms. Patients reported reduced dyspnea, greater activity tolerance, and improved quality of life. Again, the low-dose arm lagged behind, with many patients remaining in advanced functional classes.
Biomarkers told a similar story. NT-proBNP levels fell significantly in the standard and high-dose groups, reflecting reduced right ventricular strain. The low-dose group showed attenuated reductions, suggesting persistent hemodynamic burden. Together, these data harmonize with the survival outcomes: standard dosing is sufficient, higher dosing adds little, and low dosing is harmful.
Safety and Tolerability
Safety is always a concern in dose-finding trials. Interestingly, the trial revealed no major differences in serious adverse events between the standard and high-dose arms. Common side effects—headache, flushing, nasal congestion, and gastrointestinal complaints—were slightly more frequent at higher doses, but rarely led to discontinuation.
Visual disturbances, attributable to off-target inhibition of retinal PDE6, were reported more often in the high-dose group. However, these effects were transient and not associated with permanent impairment. Hypotension episodes were infrequent and manageable. Importantly, no unexpected safety signals emerged even at the highest dose.
In contrast, the low-dose group paradoxically reported fewer side effects but suffered from higher mortality. This illustrates a sobering truth: tolerability does not equate to efficacy. A drug can be well tolerated yet catastrophically ineffective when underdosed. Clinicians must therefore balance comfort with survival, a principle especially relevant in life-limiting conditions like PAH.
Implications for Clinical Practice
The clinical message of this trial is both simple and profound: 20 mg three times daily remains the gold standard for sildenafil in PAH.
For physicians who had escalated dosing in pursuit of marginal gains, the evidence now suggests futility. Patients do not live longer or function better at 80 mg, though they may endure more side effects. At the same time, attempts to reduce dose—whether for cost, tolerability, or patient preference—should be strongly discouraged, as underdosing is associated with excess mortality.
The results also strengthen regulatory guidance. While practice often diverges from label recommendations, mortality data should recalibrate prescribing habits. Insurers and health systems now have robust evidence to standardize reimbursement and formulary decisions at the optimal dose.
Finally, the trial highlights the importance of dose-ranging studies in chronic diseases. Too often, once a drug is approved, dosing becomes dogma rather than an evidence-based variable. This study demonstrates that dose matters, not only for efficacy and safety, but for survival itself.
Broader Lessons in PAH Management
Beyond sildenafil, the trial offers broader lessons for the management of PAH.
First, it reinforces the principle that combination therapy remains central. While sildenafil provides meaningful survival benefit, PAH is a multifactorial disease requiring multimodal therapy. Endothelin receptor antagonists, prostacyclin analogues, and guanylate cyclase stimulators all have roles to play. Sildenafil dosing must be optimized within this broader therapeutic orchestra.
Second, it illustrates the challenge of personalized medicine in PAH. While pharmacogenomic differences may exist, this trial suggests that the survival benefit of standard dosing is broadly generalizable. Attempts to individualize dose upward did not confer advantage, suggesting that biology converges toward a ceiling effect across populations.
Third, the trial underscores the need for ongoing long-term studies. While the 16-week data were compelling, PAH is a lifelong disease. Understanding how dosing influences survival over years, rather than months, will remain a critical area for future research.
Conclusion
The Hoeper 2024 multicenter trial delivered a definitive verdict on sildenafil dosing in PAH. By comparing low, standard, and high doses head-to-head, the investigators revealed a clear hierarchy: low doses kill, standard doses save, and high doses add no survival benefit.
This conclusion crystallizes years of clinical debate and real-world improvisation. For clinicians, it provides clarity and confidence: prescribe 20 mg three times daily, neither less nor more. For regulators, it justifies current label recommendations. For patients, it offers reassurance that the simplest regimen is also the best.
In the relentless battle against PAH, sildenafil remains a cornerstone. But this trial reminds us that even cornerstones require careful placement. Too little support, and the structure collapses; too much, and the excess adds nothing. The art of medicine lies not only in choosing the right drug but in prescribing it at the right dose.
FAQ
1. Why is 20 mg three times daily considered the optimal sildenafil dose in PAH?
Because survival, functional outcomes, and biomarkers all plateau at this dose. Higher doses add side effects without prolonging life, and lower doses significantly increase mortality.
2. Can patients with PAH safely reduce sildenafil dosing for cost or tolerability reasons?
No. The trial showed that reducing to 5 mg three times daily led to higher death rates. Dose reduction is not safe and should not be practiced.
3. Do higher doses of sildenafil ever have a role in PAH?
Not for mortality or functional benefit. In rare cases of combination therapy or research protocols, higher doses may be studied, but routine use at 80 mg provides no survival advantage.