Introduction
Traumatic wounds represent one of the most persistent challenges in clinical practice. From minor cuts to deep surgical injuries, wound management remains central to surgery, emergency medicine, and long-term rehabilitation. Despite centuries of progress, the “ideal” wound healing agent—fast, safe, and effective across all stages of repair—remains elusive. Dressings, antiseptics, anti-inflammatory drugs, and growth factor-based therapies each provide partial benefits, yet none adequately address the full spectrum of wound repair.
Sildenafil citrate, better known for its use in erectile dysfunction, has recently attracted attention for its nitric oxide (NO)-modulating properties. Since NO is deeply involved in vasodilation, angiogenesis, inflammation, and collagen deposition, sildenafil provides a mechanistic rationale for wound repair. While systemic sildenafil has shown benefits in surgical and ischemic wound models, its topical potential has been underexplored. A topical approach could localize the drug’s action, minimize systemic exposure, and provide a practical tool for acute wound care.
This article examines recent preclinical work on the development of sildenafil citrate-based hydrogels (SCHs). These novel formulations have been tested for dermal safety and wound healing efficacy in animal models, offering a new perspective on how a familiar drug might be repurposed for trauma medicine. The findings not only highlight promising therapeutic effects but also raise intriguing questions about how pharmacology and biomaterials can converge to advance wound care.
The Biological Basis of Wound Healing
Wound healing is a complex, tightly regulated biological process that unfolds in three overlapping phases: inflammation, proliferation, and remodeling. Each stage requires specific cellular players and signaling cascades, and failure at any point leads to delayed or pathological healing.
The inflammatory phase begins immediately after injury. Vascular constriction is followed by platelet aggregation and the release of cytokines, which recruit neutrophils and macrophages. These cells clear debris, release antimicrobial molecules, and set the stage for tissue regeneration. However, excessive inflammation can be detrimental, leading to tissue damage and chronic wounds.
Next comes the proliferative phase, marked by fibroblast migration, angiogenesis, and extracellular matrix deposition. Endothelial cells form new capillaries, fibroblasts secrete collagen, and keratinocytes migrate to close the wound surface. A balanced supply of oxygen, nutrients, and growth factors is essential at this stage.
Finally, the remodeling phase involves collagen maturation, reorganization of the extracellular matrix, and strengthening of the tissue. Tensile strength progressively increases as type III collagen is replaced by type I, and skin appendages such as hair follicles may regenerate. This phase can last weeks to months depending on wound severity.
Nitric oxide plays roles in all three phases. It promotes vasodilation in the inflammatory stage, supports angiogenesis in the proliferative stage, and regulates collagen synthesis during remodeling. Agents that can stimulate NO production, therefore, hold broad therapeutic promise. Sildenafil citrate, by inhibiting phosphodiesterase type-5 (PDE-5) and enhancing the NO–cGMP pathway, emerges as a candidate capable of influencing every stage of wound healing.
Why Hydrogels? The Case for Topical Delivery
Formulation matters as much as the active drug. Hydrogels—water-rich polymer networks—are widely regarded as ideal vehicles for topical therapy. Their biocompatibility, ability to maintain a moist environment, and ease of application make them particularly suitable for wound management.
Topical sildenafil delivery through hydrogels provides several advantages. First, it allows local action, concentrating the drug where it is needed and minimizing systemic absorption. This is crucial for a drug like sildenafil, whose systemic effects (hypotension, headache, flushing) may be undesirable in wound patients. Second, hydrogels maintain a hydrated wound bed, which is known to accelerate re-epithelialization, reduce infection risk, and support enzymatic activity. Finally, hydrogels can be engineered for controlled drug release, ensuring sustained exposure to therapeutic levels.
The development of sildenafil citrate hydrogels thus combines pharmacological logic with material science, offering a dual advantage: an effective molecule delivered through an optimal platform.
Formulation and Characterization of Sildenafil Citrate Hydrogels
Researchers prepared sildenafil citrate hydrogels in three concentrations: 3% (SF1), 5% (SF2), and 10% (SF3). Carbopol served as the gelling agent, and stability tests confirmed that all formulations maintained neutral pH, homogeneity, and spreadability over time. Unlike creams or sprays, which suffer from stability and adhesion problems, the hydrogel formulations were robust under varied storage conditions.
Scanning electron microscopy (SEM) revealed smooth and uniform surfaces, with minor imperfections typical of polymer networks. Encapsulation efficiency averaged over 50%, ensuring that sufficient drug was retained within the gel matrix for therapeutic delivery. In vitro release studies demonstrated rapid and pH-independent drug release, with more than 50% of sildenafil released within 30 minutes and maximal release by two hours.
From a pharmaceutical standpoint, these results indicate formulations that are both stable and capable of delivering the active drug efficiently—critical prerequisites before moving to biological evaluation.
Dermal Toxicity and Safety Profile
Safety is paramount for any topical product. The hydrogel formulations underwent a 21-day repeated dermal toxicity study in Sprague-Dawley rats, following OECD guidelines. Key parameters included general health, body weight, feed consumption, skin irritation, hematology, and biochemistry.
Results were reassuring. None of the sildenafil hydrogels produced significant changes in body weight or feeding behavior. Hematological indices (WBC, RBC, hemoglobin, platelets) remained within physiological ranges. Biochemical markers of liver function (SGOT, SGPT, ALP) showed no concerning deviations, except for mild, transient changes in placebo groups unrelated to sildenafil.
The primary irritation index (PII) for all formulations fell within the “non-irritant” range. Interestingly, higher concentrations (5% and 10%) caused less irritation than the 3% gel, suggesting a paradoxical protective effect at effective doses. This counterintuitive result may reflect sildenafil’s antioxidant activity, which neutralizes free radicals and stabilizes the local environment.
Taken together, these findings demonstrate that sildenafil citrate hydrogels are safe for dermal use and do not provoke systemic toxicity or local irritation when applied repeatedly.
Wound Healing Efficacy: Preclinical Insights
The real test of any wound healing formulation lies in its ability to accelerate and improve tissue repair. The sildenafil hydrogels were evaluated in an excision wound model in rats, comparing untreated controls, a standard treatment (povidone-iodine), and the three sildenafil formulations.
Wound Contraction and Re-epithelialization
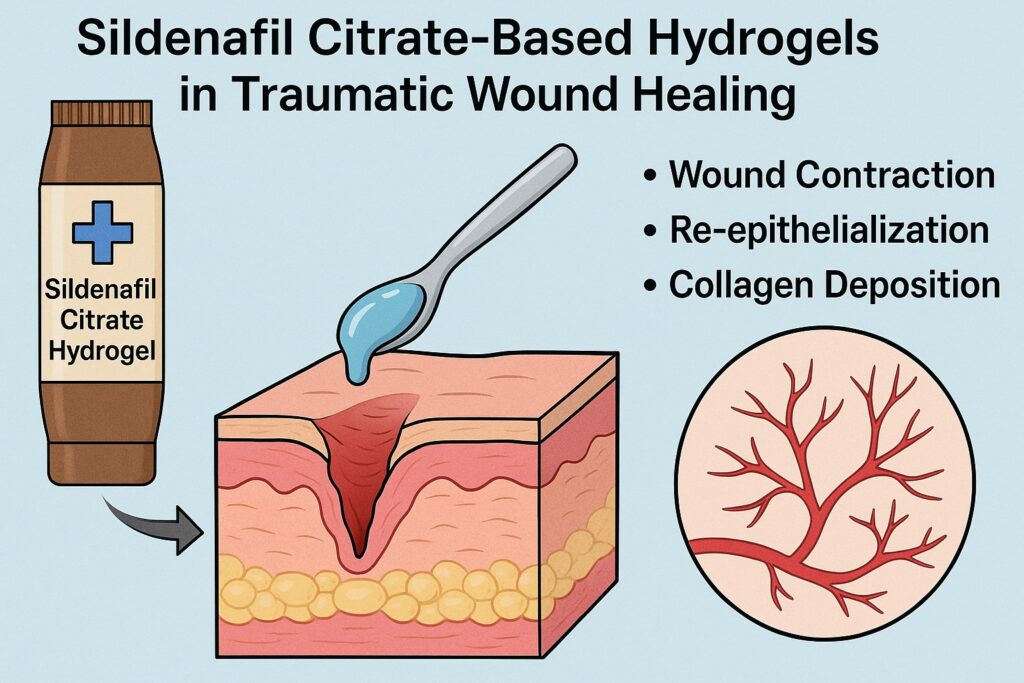
By day 7, the 5% and 10% hydrogels achieved nearly 30% greater wound contraction than controls, a trend that persisted through day 14. Re-epithelialization rates were equally impressive: 65–66% for SF2 and SF3 compared to 38% in untreated controls. The 3% gel showed intermediate results, suggesting a clear dose-response relationship.
Tensile Strength
Mechanical testing of healed tissue revealed that sildenafil gels enhanced tensile strength in a concentration-dependent manner. SF2 and SF3-treated wounds were significantly stronger than untreated or standard-treated wounds. Stronger scar tissue not only reflects better collagen deposition but also reduces the risk of wound reopening.
Biochemical Markers
Sildenafil treatment increased nitric oxide production, hydroxyproline content, and collagen levels, all in dose-dependent fashion. Hydroxyproline, a marker of collagen turnover, was significantly higher in SF2 and SF3 groups, correlating with improved tensile strength. Protein and DNA contents also increased, reflecting active cellular proliferation and tissue regeneration.
Histopathology
Microscopic analysis offered the most compelling evidence. By day 7, sildenafil-treated wounds showed early re-epithelialization, abundant granulation tissue, and even initial hair follicle regeneration. By day 14, collagen fibers were compactly organized, the epidermis was thicker, and neovascularization was evident. Compared to untreated controls, where healing lagged with persistent inflammation, sildenafil gels demonstrated accelerated and organized tissue repair.
The Optimal Formulation: Why 5% Works Best
While both 5% (SF2) and 10% (SF3) gels performed well, the 5% formulation emerged as the optimal balance. It achieved wound closure rates comparable to the higher concentration while maintaining minimal irritation and efficient NO stimulation. Increasing from 5% to 10% added little benefit but increased formulation complexity. Thus, 5% sildenafil hydrogel stands out as a practical candidate for future development.
Broader Implications and Future Perspectives
Repurposing sildenafil citrate as a wound healing agent exemplifies the creative rethinking of established drugs. The findings suggest several broader implications:
- Topical sildenafil could benefit diverse wound types, from surgical incisions to burns and chronic ulcers.
- NO-modulating hydrogels may serve as a platform for combining sildenafil with other bioactive agents such as growth factors, antimicrobial peptides, or antioxidants.
- Translation to human application requires careful clinical trials, addressing not only efficacy but also systemic absorption, dosing frequency, and long-term safety.
Challenges remain. Human skin differs from rodent skin in thickness, elasticity, and healing dynamics. Regulatory hurdles will require robust toxicology data, standardized formulations, and reproducible manufacturing processes. Furthermore, while the preclinical evidence is strong, clinical validation is indispensable before such gels can enter routine practice.
Conclusion
The development of sildenafil citrate-based hydrogels represents a promising step in wound care innovation. By combining the pharmacological power of nitric oxide modulation with the practical advantages of hydrogels, researchers have created a formulation that accelerates wound contraction, enhances re-epithelialization, strengthens scar tissue, and promotes organized collagen deposition. Safety studies confirm that the gels are non-toxic and well-tolerated, paving the way for translation into human trials.
Among the tested formulations, the 5% hydrogel strikes the ideal balance of efficacy and safety. If future clinical studies confirm these findings, sildenafil hydrogels may offer clinicians a new tool to treat traumatic wounds more effectively, while patients benefit from faster recovery and reduced complications.
In an era where drug repurposing and biomaterial science converge, sildenafil citrate hydrogels remind us that sometimes the most innovative solutions arise from familiar molecules, reimagined for new frontiers in medicine.
FAQ
1. How does sildenafil help in wound healing?
Sildenafil enhances nitric oxide signaling by inhibiting PDE-5, leading to vasodilation, angiogenesis, collagen deposition, and reduced inflammation. These mechanisms collectively accelerate wound closure and tissue regeneration.
2. Is topical sildenafil safe for the skin?
Preclinical studies show that sildenafil hydrogels are safe, non-irritant, and do not cause systemic toxicity when applied repeatedly. Human trials are needed to confirm these findings, but animal data are reassuring.
3. Which concentration of sildenafil gel works best?
The 5% formulation demonstrated optimal results, providing faster healing and stronger tissue with minimal irritation. The 10% gel offered no significant advantage, making 5% the practical choice for further development.