Introduction
Testicular torsion remains one of the most feared emergencies in andrology and urology. When a testis twists upon the spermatic cord, it creates an acute ischemic state that rapidly endangers tissue viability. Prompt detorsion is often lifesaving for the affected testis, yet even with surgical correction, significant long-term complications may occur. One particularly perplexing phenomenon is the injury observed in the contralateral, non-twisted testis. This paradoxical damage is believed to be linked to ischemia-reperfusion injury and complex systemic responses that extend beyond the site of the torsion.
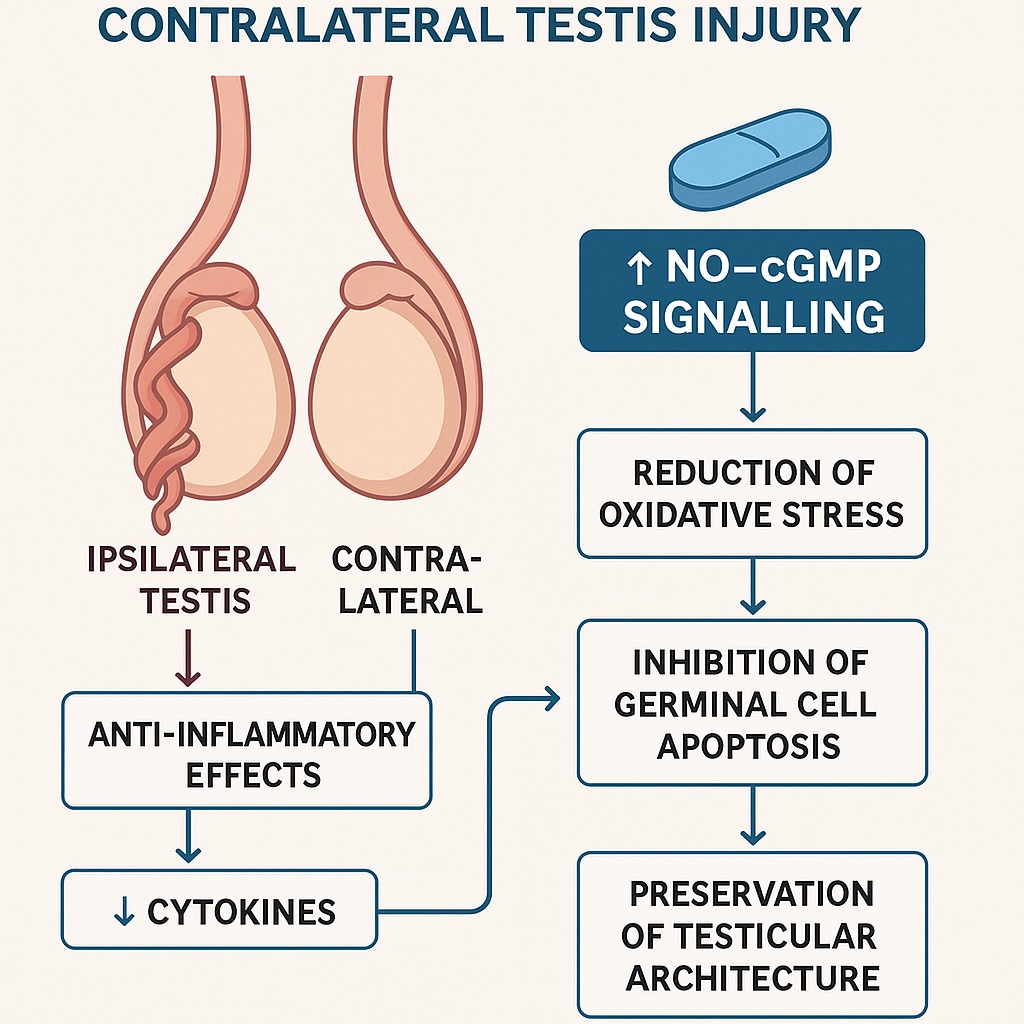
In recent years, sildenafil citrate, widely known for its role in erectile dysfunction, has been investigated for its cytoprotective, anti-inflammatory, and antioxidant properties. These effects stem largely from its ability to modulate the nitric oxide–cyclic guanosine monophosphate (NO–cGMP) pathway. Experimental studies suggest that sildenafil may offer a protective shield against contralateral testicular damage following unilateral torsion and detorsion. The idea is not simply therapeutic opportunism but rests upon a robust mechanistic rationale.
This article explores the interplay between testicular torsion, contralateral testis injury, and the potential for sildenafil to modify outcomes. We will examine the underlying biology, summarize the experimental evidence, and consider how these findings could reshape future management strategies.
Testicular Torsion and the Problem of Contralateral Injury
The classical teaching regarding testicular torsion focuses on the twisted side, where ischemia develops rapidly. Spermatogenic cells are highly vulnerable to oxygen deprivation, and necrosis can occur within hours if reperfusion is not achieved. Surgical detorsion aims to restore perfusion, but paradoxically, reperfusion itself can intensify tissue damage by unleashing reactive oxygen species (ROS), inflammatory mediators, and apoptotic signaling cascades.
The contralateral testis often demonstrates signs of histological and functional compromise after torsion-detorsion, even though it was never directly deprived of blood flow. Several hypotheses have been proposed to explain this phenomenon:
- Sympathetic reflexes: Neural cross-talk may provoke vasoconstriction and hypoperfusion in the contralateral testis.
- Autoimmune mechanisms: Disruption of the blood-testis barrier on the affected side may expose antigens that provoke systemic immune responses against germ cells in both testes.
- Systemic oxidative stress: Ischemia-reperfusion generates free radicals and cytokines that can circulate and harm distant tissues.
Clinically, this translates into reduced fertility potential, decreased sperm quality, and in some cases, bilateral testicular atrophy despite successful detorsion on one side. Thus, the contralateral testis represents an underappreciated victim of torsion events, and its protection is a legitimate therapeutic target.
Sildenafil Citrate: More Than an Erectile Dysfunction Drug
Sildenafil citrate is a selective phosphodiesterase type 5 (PDE5) inhibitor. Its primary mechanism is the prevention of cGMP degradation, thereby amplifying nitric oxide-mediated vasodilation in penile tissue. Yet, the NO–cGMP axis is ubiquitous across vascular and non-vascular tissues, granting sildenafil a much broader biological impact.
In preclinical research, sildenafil has demonstrated several properties that may be beneficial in the context of testicular torsion-detorsion:
- Anti-apoptotic activity: By stabilizing cGMP levels, sildenafil can inhibit pro-apoptotic pathways in germ cells.
- Antioxidant effects: Indirect upregulation of antioxidant enzymes reduces the burden of ROS after reperfusion.
- Anti-inflammatory action: Sildenafil decreases neutrophil infiltration and dampens cytokine release, reducing tissue-level inflammation.
- Improved microcirculation: Vasodilation extends beyond penile tissue, potentially improving perfusion in ischemia-prone organs.
These actions suggest that sildenafil may limit both the local and systemic damage associated with reperfusion injury, making it a plausible candidate for testicular protection.
Experimental Evidence: Sildenafil in Testicular Torsion Models
Animal studies provide the foundation for our current understanding of sildenafil’s role in testicular torsion. In controlled experimental designs, unilateral torsion is induced in rodents, followed by detorsion to simulate clinical intervention. Sildenafil is then administered, and both ipsilateral and contralateral testes are evaluated histologically and biochemically.
The findings have been consistent and revealing:
- Reduction in Germ Cell Apoptosis
Sildenafil-treated groups showed significantly lower rates of germ cell apoptosis in the contralateral testis. TUNEL assays and caspase-3 activity measurements confirmed attenuation of apoptotic signaling. - Improvement in Histological Architecture
Histological examination revealed preserved seminiferous tubule structure and reduced interstitial edema in the sildenafil group compared to untreated controls. - Decrease in Oxidative Stress Markers
Malondialdehyde (MDA), a marker of lipid peroxidation, was significantly lower in sildenafil-treated testes. Conversely, activities of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase were elevated. - Modulation of Inflammatory Response
Sildenafil reduced infiltration of neutrophils and downregulated pro-inflammatory cytokines, which are otherwise elevated after torsion-detorsion. - Preservation of Spermatogenesis
Importantly, spermatogenic activity in the contralateral testis was better preserved in sildenafil-treated animals, suggesting potential long-term fertility benefits.
The convergence of these findings across multiple experimental setups builds a compelling case for sildenafil’s protective role.
Mechanistic Underpinnings: How Sildenafil Confers Protection
The beneficial effects of sildenafil in the contralateral testis cannot be attributed to a single pathway. Rather, they represent a convergence of complementary mechanisms:
Modulation of NO–cGMP Signaling
Sildenafil’s core mechanism is the inhibition of PDE5, resulting in elevated cGMP levels. This prolongs nitric oxide signaling, which not only promotes vasodilation but also inhibits platelet aggregation and reduces leukocyte adhesion. Enhanced cGMP signaling also stabilizes mitochondrial function, a key factor in preventing apoptosis.
Reduction of Oxidative Stress
Reperfusion floods tissues with oxygen, paradoxically leading to ROS generation. Sildenafil reduces this oxidative burden by enhancing antioxidant defense systems. Studies demonstrate higher SOD and catalase activity, suggesting that sildenafil primes the cellular environment to neutralize ROS more effectively.
Anti-Inflammatory Effects
By attenuating NF-κB activation and cytokine release, sildenafil curtails the inflammatory cascade that often exacerbates tissue injury. Neutrophil infiltration, a hallmark of reperfusion damage, is significantly reduced in treated groups.
Preservation of Mitochondrial Integrity
Mitochondria are central to both energy production and apoptotic signaling. Sildenafil helps preserve mitochondrial membrane potential, reducing cytochrome c release and preventing activation of downstream caspases.
Together, these mechanisms create a protective microenvironment in the contralateral testis, mitigating the secondary insults of ischemia-reperfusion.
Clinical Implications and Future Directions
While the bulk of evidence currently resides in animal models, the implications for clinical practice are profound. Protecting the contralateral testis could change the long-term prognosis of young men who experience torsion, many of whom are in their reproductive prime.
The potential applications are several:
- Perioperative adjuvant therapy: Administering sildenafil before or immediately after detorsion surgery may mitigate reperfusion injury.
- Fertility preservation: Protection of spermatogenesis in the contralateral testis could maintain fertility potential, a crucial concern for adolescents and young adults.
- Broader ischemia-reperfusion contexts: Lessons from torsion-detorsion may extend to other ischemic conditions where sildenafil could be protective, such as renal ischemia or myocardial reperfusion injury.
Of course, clinical translation requires careful consideration. Dosing regimens, timing of administration, and potential side effects in pediatric populations must be rigorously studied. Moreover, the psychosocial connotation of sildenafil as a “sexual medicine” may complicate its acceptance in young male patients unless communicated carefully.
Challenges and Considerations
Despite the encouraging evidence, several challenges remain:
- Species differences: Rodent models may not perfectly mimic human testicular physiology. Translation must be cautious.
- Timing of intervention: The window of opportunity for sildenafil to be effective is uncertain; delayed administration may blunt its protective effects.
- Long-term outcomes: While short-term preservation of histology is promising, long-term fertility data are lacking.
- Ethical considerations: Using sildenafil in prepubertal or adolescent patients raises unique ethical and practical issues that must be carefully addressed.
These caveats highlight the need for well-designed clinical trials before sildenafil can be adopted as a standard adjunct in torsion management.
Conclusion
Testicular torsion is a urological emergency that imperils not only the affected testis but also its contralateral partner through complex systemic mechanisms. The contralateral injury, long overlooked, has significant implications for fertility and endocrine function. Sildenafil citrate, through its multifaceted modulation of the NO–cGMP pathway, offers a promising avenue for protection.
By reducing apoptosis, curbing oxidative stress, and dampening inflammation, sildenafil preserves contralateral testicular integrity in experimental models. While clinical translation is still on the horizon, the conceptual foundation is strong. If corroborated in human studies, sildenafil could be repositioned as not only a treatment for erectile dysfunction but also as a guardian of testicular health in the face of ischemic injury.
FAQ
1. Why does the contralateral testis get damaged during torsion-detorsion if it is not directly twisted?
Contralateral damage arises from systemic responses such as oxidative stress, autoimmune activation, and sympathetic reflexes. These processes extend injury beyond the torsed side, jeopardizing the supposedly “safe” testis.
2. How does sildenafil protect testicular tissue?
Sildenafil enhances NO–cGMP signaling, reduces oxidative stress, dampens inflammation, and preserves mitochondrial function. Together, these mechanisms prevent germ cell apoptosis and maintain testicular architecture.
3. Could sildenafil realistically be used in clinical management of testicular torsion?
Potentially, yes. Early administration during or after detorsion surgery might protect the contralateral testis and preserve fertility. However, human trials are necessary to establish safety, dosing, and efficacy before clinical adoption.