Introduction: The Hidden Cardiac Toll of Burn Injury
Severe burn injuries are among the most devastating forms of trauma, both physically and physiologically. While public health narratives often focus on skin healing and infection prevention, an equally critical but less visible consequence lies deep within the thoracic cavity—the heart itself. Burn injury initiates a cascade of inflammatory, oxidative, and fibrotic events that collectively impair cardiac function. The resulting condition, known as burn-induced cardiomyopathy, can dramatically increase morbidity and mortality in critically burned patients.
Traditionally, cardiac dysfunction in burn victims has been attributed to hypovolemia, hypermetabolism, and systemic inflammation. However, recent mechanistic studies have illuminated a more intricate molecular picture: one dominated by the PDE5A–cGMP–PKG signaling pathway. The enzyme phosphodiesterase type 5A (PDE5A), best known for its role in erectile physiology, also resides in cardiomyocytes where it regulates cyclic guanosine monophosphate (cGMP)—a key second messenger responsible for vascular relaxation and myocardial contractility.
When burns strike, this pathway becomes pathologically disrupted. The University of Texas Medical Branch team, led by Wen, Cummins, and Radhakrishnan (2020), demonstrated that PDE5A is overexpressed following major thermal trauma, precipitating oxidative stress, fibrosis, and contractile failure. Their study further revealed a surprising hero in this narrative—sildenafil, the same molecule celebrated for treating erectile dysfunction. Through rigorous experimentation, they showed that sildenafil can restore cardiac function after severe burns by rebalancing intracellular signaling, reducing inflammation, and preventing oxidative injury.
The Pathophysiological Chain Reaction of Severe Burns
Burn injury is not confined to the dermis—it triggers a systemic catastrophe that disrupts virtually every organ. Within hours of the insult, inflammatory mediators surge, endothelial barriers weaken, and tissue perfusion becomes erratic. In the myocardium, this translates into depressed contractility, mitochondrial dysfunction, and cellular apoptosis.
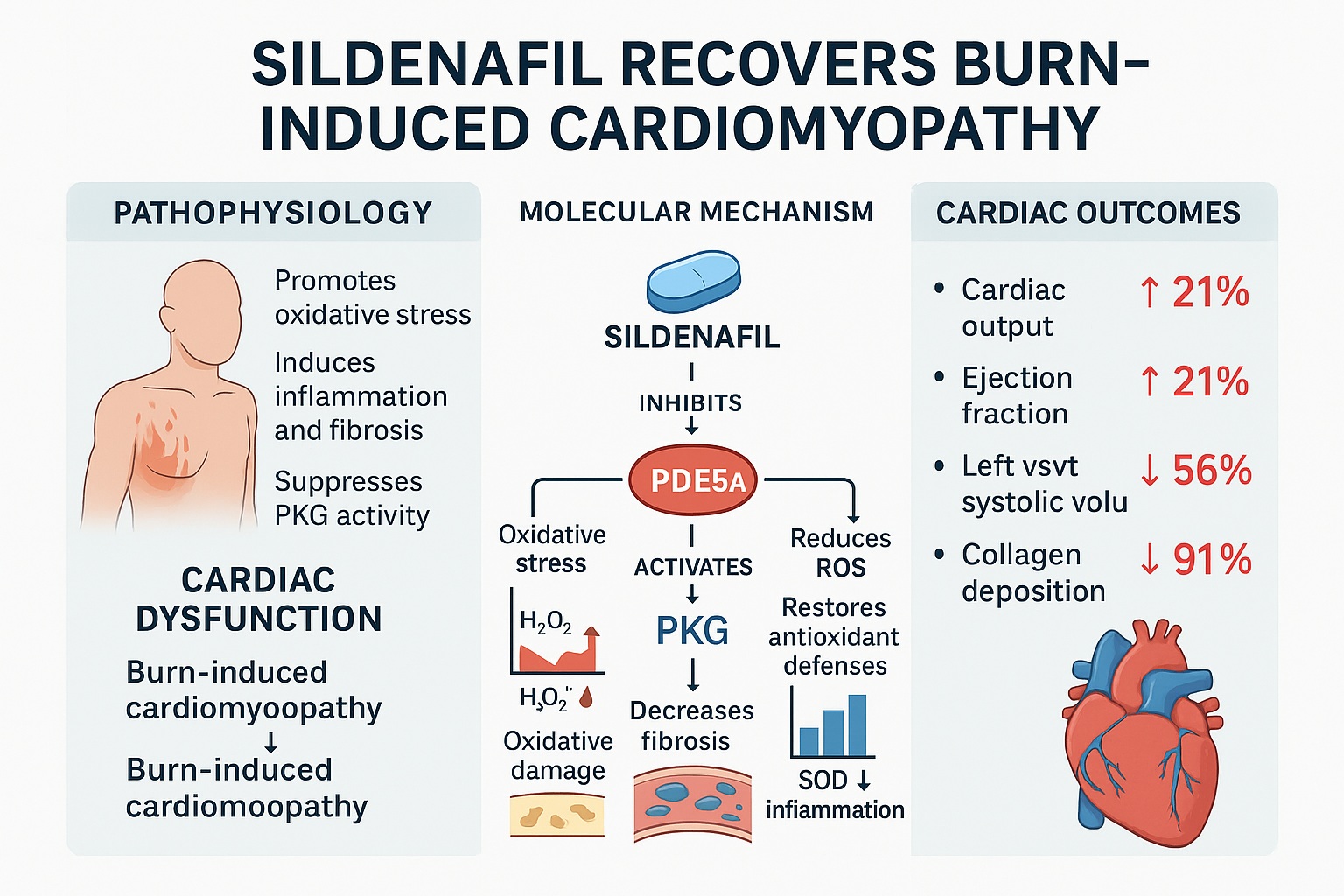
Central to this decline is the excessive activation of PDE5A, which hydrolyzes cGMP into its inactive form. Under normal conditions, cGMP activates protein kinase G (PKG1α), a guardian enzyme that preserves cardiac tone and limits fibrotic signaling. When cGMP levels fall, PKG1α activity collapses, leading to unchecked calcium influx, endothelial dysfunction, and hypercontractile stress on cardiomyocytes.
The consequences are devastating: reduced cardiac output, impaired stroke volume, and left ventricular dilation—all hallmarks of acute heart failure. The study hypothesized that inhibiting PDE5A could interrupt this destructive cycle, restoring cGMP signaling and normalizing myocardial performance. Sildenafil, a selective PDE5A inhibitor, became the pharmacological probe to test this theory.
Study Overview: How the Experiment Was Designed
Using male Sprague–Dawley rats, the researchers simulated a 60% total body surface area (TBSA) scald burn, a model designed to replicate severe thermal trauma in humans. The animals were divided into four groups:
- Sham (control)
- Sham + sildenafil
- Burn (24 hours post-burn)
- Burn + sildenafil
Each burn-subjected animal received immediate fluid resuscitation, with or without sildenafil at 2 mg/kg. After 24 hours, the hearts were harvested for an array of molecular, biochemical, and echocardiographic analyses.
The methodology combined nano LC–MS/MS proteomics, qPCR for gene expression, ELISA assays for cGMP and PKG activity, and echocardiography for cardiac performance metrics. Additional histology and oxidative stress assays quantified fibrosis, inflammation, and free radical burden.
The comprehensiveness of this design allowed the researchers to trace sildenafil’s effects from gene expression all the way to organ function—creating a complete mechanistic portrait of its cardioprotective role.
The Molecular Domino: PDE5A, cGMP, and PKG in the Burned Heart
The findings were unequivocal. Within 24 hours of a major burn, cardiac PDE5A expression tripled, while cGMP levels fell by 50% and PKG activity dropped by 32%. Concurrently, the expression of PKG-regulated genes such as IRAG, PLB, RGS2, and MYTP plummeted, while RhoA, a pro-fibrotic GTPase, doubled.
When sildenafil was administered, these aberrations were reversed. PDE5A mRNA normalized, cGMP surged back to physiological levels, and PKG activity recovered. This molecular rescue reinstated the proper phosphorylation balance across contractile proteins, restoring myocardial rhythm and tone.
Essentially, sildenafil reactivated the “molecular brakes” of cardiac stress signaling. By suppressing excessive PDE5A, it allowed cGMP and PKG to reclaim their regulatory authority—protecting the heart from self-destruction.
Restoring the Beating Engine: Echocardiographic Evidence
At the functional level, burns drastically reduced key indicators of cardiac performance:
- Cardiac Output (CO) ↓ 21%
- Ejection Fraction (EF) ↓ 21%
- Stroke Volume (SV) ↓ 24%
- Left Ventricular Wall Thickness ↓ 32%
- Fractional Shortening (FS) ↓ 39%
Moreover, the left ventricular systolic volume increased by 126%, signaling acute dilation and reduced contractile efficiency.
Following sildenafil treatment, every parameter returned nearly to sham control levels. The drug not only restored systolic performance but also prevented early ventricular remodeling. These data offer compelling preclinical evidence that PDE5A inhibition can rescue cardiac contractility even in the midst of post-burn inflammation and metabolic stress.
The Fibrotic Heart: How Sildenafil Blocks Collagen Storms
Histological analysis revealed that severe burns triggered a seven-fold increase in myocardial collagen deposition, accompanied by surges in fibrotic markers including ANP, BNP, COL1A2, COL3A2, αSMA, and ACTA. This profibrotic storm not only stiffened cardiac tissue but also compromised diastolic compliance.
Treatment with sildenafil alleviated over 90% of collagen accumulation, effectively halting fibrogenesis. Trichrome-stained heart sections confirmed a dramatic reduction in blue-stained collagen bands, while qPCR demonstrated normalization of fibrotic gene expression.
Mechanistically, sildenafil’s suppression of TGF-β and RhoA signaling likely contributed to this anti-fibrotic effect. The findings suggest that the PDE5A–cGMP–PKG pathway regulates not just contractility but also the extracellular matrix architecture of the heart—a revelation that positions sildenafil as an anti-fibrotic agent in its own right.
Quelling the Fire Within: Anti-Inflammatory Effects of Sildenafil
Post-burn myocardium displayed diffuse inflammatory infiltrates with elevated expression of inflammatory mediators NF-κB (RELA), IL-18, and TGF-β. These cytokines are notorious for amplifying tissue injury and initiating fibrosis.
Sildenafil significantly suppressed all three markers. NF-κB activity dropped by 80%, and IL-18 and TGF-β expression returned to baseline. Histological scores of myocardial inflammation decreased from 2.8 (moderate-severe) to 0.9 (mild).
This anti-inflammatory profile underscores sildenafil’s multi-dimensional protection—not only as a hemodynamic modulator but also as a molecular shield against cytokine toxicity.
Redox Rebalancing: Oxidative Stress and Antioxidant Defense
Burn injury generated a biochemical tempest of reactive oxygen species (ROS). In the study:
- Hydrogen peroxide (H₂O₂) increased 16-fold
- Malondialdehyde (MDA) rose 3.4-fold
- Protein carbonyl adducts surged 19-fold
Simultaneously, antioxidant defenses collapsed—total antioxidant capacity fell 60%, total SOD activity 87%, and Cu/Zn-SOD 22%.
Sildenafil normalized all these markers. H₂O₂ and MDA levels dropped to near-baseline, and antioxidant enzyme activity was restored. These effects likely stem from sildenafil’s indirect stimulation of the NFE2L2 antioxidant response pathway, enhancing the cell’s intrinsic capacity to neutralize ROS.
By restoring redox balance, sildenafil protected mitochondrial integrity, improved energy metabolism, and prevented apoptotic signaling within cardiac cells.
Gene-Level Modulation: Repairing the Heart’s Transcriptional Machinery
Beyond the visible physiological recovery, sildenafil also recalibrated the genetic landscape of the burned heart. mRNA analyses revealed that sildenafil restored the expression of key genes involved in calcium handling, myosin regulation, and intracellular signaling:
- IRAG (inositol trisphosphate receptor-associated cGMP kinase substrate) — normalized
- PLB (phospholamban) — restored, improving calcium homeostasis
- RGS2 and MYTP — reinstated, stabilizing G-protein signaling and contractile coordination
These changes collectively reestablished the molecular synchrony necessary for efficient cardiac contraction. Without such repair, the myocardium remains vulnerable to arrhythmias and long-term failure—a fate sildenafil effectively prevented.
The Broader Implications: From the Heart to Clinical Horizons
This study redefines sildenafil’s pharmacological territory. Originally designed as an anti-anginal drug and repurposed for erectile dysfunction, sildenafil now demonstrates clear cardioprotective potential in inflammatory and oxidative settings beyond its vascular niche.
Clinical parallels already exist: PDE5A inhibitors have shown benefits in diabetic cardiomyopathy, pulmonary hypertension, and ischemic heart failure. What differentiates the burn model is the acute, systemic inflammatory insult, which sildenafil was able to counteract within 24 hours—an extraordinarily rapid timeframe for cardiac recovery.
These findings may open new avenues for early post-burn intervention protocols, especially for patients at risk of cardiovascular instability. The prospect of using a well-characterized, FDA-approved molecule in this context is both scientifically and clinically appealing.
The Mechanistic Symphony: A Unified Model
When the data are synthesized, a coherent mechanism emerges:
- Burn injury triggers PDE5A overexpression → cGMP depletion → PKG suppression.
- Reduced PKG permits unchecked fibrosis, inflammation, and ROS accumulation.
- Sildenafil inhibits PDE5A → restores cGMP and PKG → activates antioxidant and anti-fibrotic pathways.
- Result: Normalized cardiac performance, reduced oxidative damage, preserved mitochondrial health.
This elegant model illustrates how sildenafil transforms from a vasodilator into a cellular reprogrammer, reinstating physiological balance at multiple biological levels—from nucleotide metabolism to gene expression.
Clinical Perspective: Could Sildenafil Be the Next Cardioprotective Adjunct in Burn Care?
Although preclinical, the implications are tangible. Current burn management primarily addresses fluid resuscitation, infection control, and wound care. Cardiac protection is often reactive rather than preventive. The introduction of sildenafil as a pharmacological safeguard against post-burn cardiomyopathy could redefine critical care protocols.
Moreover, sildenafil’s safety profile and affordability make it a pragmatic candidate for translational studies. Low-dose regimens might complement standard resuscitation, reducing mortality from cardiac complications—a leading cause of death in extensive burns.
Still, careful human trials are needed. Differences in pharmacokinetics, systemic inflammation, and co-morbid conditions warrant precise dose optimization and timing strategies.
Limitations and Future Directions
While the study’s findings are compelling, they represent an early stage of translational research. Several limitations remain:
- The experiment was conducted only in male rats; gender-based physiological differences require evaluation.
- The 24-hour observation period captures acute, not chronic, cardiac outcomes.
- The molecular interplay between PDE5A inhibition and adrenergic signaling after burns remains incompletely understood.
Future studies should examine long-term cardiac remodeling, test alternative PDE5A inhibitors, and assess synergistic effects with antioxidant or anti-inflammatory therapies.
Conclusion: A Familiar Drug, a New Frontier
The study by Wen and colleagues illuminates a fascinating convergence of cardiovascular pharmacology and burn medicine. It reveals that sildenafil—beyond its notoriety as a “blue pill” for sexual health—possesses powerful cardioprotective properties capable of reversing burn-induced heart failure.
By targeting the PDE5A–cGMP–PKG pathway, sildenafil restores the heart’s molecular rhythm, curtails fibrosis, quenches inflammation, and rejuvenates antioxidant defenses. In doing so, it transforms the devastated post-burn myocardium into a recovering organ capable of functional resilience.
In a broader sense, this research underscores a timeless principle in medicine: mechanisms once thought to serve one system often hold the key to healing another. The same molecule that restores blood flow in one context can restore hope in another—proving that pharmacology, like physiology, is beautifully interconnected.
FAQ: Understanding Sildenafil’s Role in Burn-Induced Heart Dysfunction
1. How does sildenafil protect the heart after a burn injury?
Sildenafil inhibits the enzyme PDE5A, which is overactivated after severe burns. By preventing cGMP breakdown, it restores PKG activity, reducing oxidative stress, inflammation, and fibrosis—ultimately preserving cardiac function.
2. Is sildenafil currently used for treating burn-induced cardiomyopathy in humans?
Not yet. The findings are preclinical, based on rat models. However, given sildenafil’s established safety record, clinical translation is plausible pending human trials.
3. Could sildenafil interfere with other cardiovascular medications?
Potentially, yes. As a vasodilator, sildenafil can interact with nitrates and antihypertensive agents, leading to hypotension. Any future use in burn patients would require cautious hemodynamic monitoring and dose calibration.