Introduction
Since its dramatic debut in the late 1990s, sildenafil citrate—better known under its brand name Viagra—has reshaped the management of erectile dysfunction. What began as a compound designed to investigate cardiovascular pathways quickly became a blockbuster medication that has restored intimacy for millions. Yet, beneath this cultural success story lies an ongoing medical debate: is sildenafil safe for patients with coronary artery disease?
Concerns emerged almost immediately after sildenafil’s release. The drug’s vasodilatory effects, mediated by phosphodiesterase type 5 (PDE5) inhibition and amplified cyclic guanosine monophosphate (cGMP) signaling, might in theory trigger “coronary steal,” worsen ischemia, or precipitate dangerous hypotension. Cardiologists were understandably cautious, particularly for patients already grappling with coronary artery stenosis.
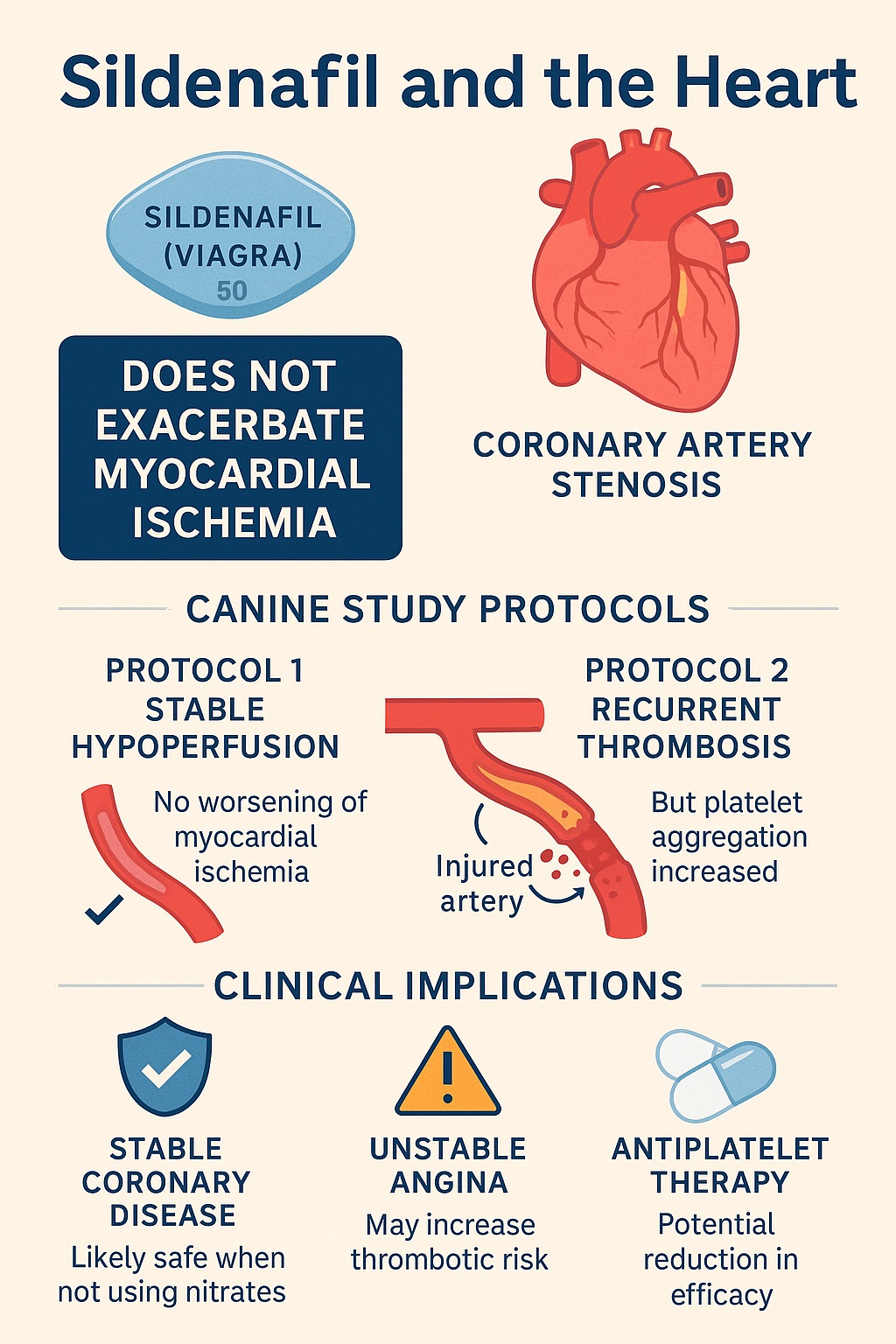
The study by Przyklenk and Kloner sought to address this concern directly by investigating sildenafil’s impact on coronary perfusion in carefully controlled canine models of stable hypoperfusion and recurrent thrombosis. Their findings offer nuanced insights—not only exonerating sildenafil in some scenarios but also raising provocative questions about its interplay with platelet biology.
In this article, I will synthesize and expand on those results, translating them into a comprehensive discussion that links basic pharmacology, experimental cardiology, and clinical implications. Our journey will examine sildenafil’s hemodynamic effects, its role in myocardial ischemia, the surprises uncovered in platelet reactivity, and the broader meaning for cardiovascular care.
Pharmacologic Foundations of Sildenafil
To understand sildenafil’s effects on the heart, one must first revisit its molecular target. PDE5 is highly expressed in the corpus cavernosum of the penis, where it regulates breakdown of cGMP. By inhibiting PDE5, sildenafil prolongs cGMP activity, enhancing nitric oxide (NO)-mediated smooth muscle relaxation and enabling penile erection.
But PDE5 is not restricted to penile tissue. It exists in vascular smooth muscle—including coronary arteries—and in platelets. This distribution raises two central questions. First, could sildenafil’s vasodilatory actions in the coronary circulation impair perfusion in stenotic arteries? Second, might its effects on platelet signaling alter thrombotic dynamics?
The answers are not straightforward. Vasodilation distal to a stenosis may theoretically improve perfusion, but it could also redistribute flow unfavorably—a phenomenon termed “coronary steal.” Platelet inhibition by cGMP elevation might sound protective, yet cellular signaling cross-talk with cyclic adenosine monophosphate (cAMP) and adenosine receptors introduces layers of complexity.
Thus, sildenafil inhabits a pharmacologic gray zone: potentially beneficial, potentially harmful, and in urgent need of empirical testing in realistic cardiovascular models.
Study Design: Canine Models of Coronary Stenosis
The investigators employed three experimental protocols, each targeting a distinct facet of coronary physiology. Using purpose-bred anesthetized dogs, they applied coronary stenoses with or without arterial injury, allowing precise control over blood flow dynamics.
Protocol 1: Stable Hypoperfusion
- A mechanical constrictor reduced coronary blood flow through an intact artery to approximately half of baseline.
- This model mimicked the clinical scenario of a narrowed artery maintaining hibernating myocardium—regions of reduced but viable tissue.
- Dogs received escalating doses of sildenafil or placebo while perfusion was monitored.
Protocol 2: Coronary Artery Injury with Stenosis
- Here, arterial damage was combined with stenosis, triggering cyclic variations in blood flow (CFVs) due to recurrent platelet-rich thrombi.
- This model simulated unstable angina, where plaque rupture and thrombosis repeatedly compromise flow.
- Again, sildenafil or placebo was administered, and perfusion patterns were observed.
Protocol 3: Platelet Aggregation in Vitro
- Platelet responsiveness was tested in blood samples exposed to sildenafil, an adenosine A2 receptor agonist, or both.
- This experiment probed whether sildenafil interfered with adenosine-mediated platelet inhibition.
Together, these protocols offered a rigorous platform to test sildenafil’s cardiovascular safety under conditions ranging from benign hypoperfusion to actively unstable coronary disease.
Key Findings: Perfusion and Hemodynamics
Stable Hypoperfusion (Protocol 1)
- Coronary blood flow fell to 50–60% of baseline after stenosis.
- Sildenafil administration did not worsen perfusion compared to placebo.
- Regional myocardial blood flow remained stable, indicating no exacerbation of ischemia.
- Sildenafil did cause transient drops in mean arterial pressure (~25 mmHg), but these resolved within minutes and did not affect flow-time area.
The takeaway: sildenafil did not provoke coronary steal or worsen perfusion in this model. Its hemodynamic impact, though noticeable, was brief and self-limited.
Recurrent Thrombosis (Protocol 2)
- In placebo animals, coronary patency improved modestly over time, reflecting adenosine-mediated platelet inhibition.
- In contrast, sildenafil-treated dogs did not experience this improvement. Flow-time area remained unchanged, and platelets appeared refractory to adenosine’s effects.
- Importantly, sildenafil did not worsen perfusion or increase thrombotic occlusion; it merely blunted the natural, protective effect of adenosine.
Here lies the paradox: sildenafil was neutral on perfusion but seemed to interfere with a physiologic antithrombotic mechanism.
Platelet Biology: A Surprise Twist
Protocol 3 provided mechanistic clues. Platelet aggregation induced by collagen was reduced by adenosine receptor stimulation, as expected. However, when sildenafil was present, this inhibitory effect vanished. In both ex vivo and in vivo contexts, sildenafil rendered platelets unresponsive to adenosine A2 receptor activation.
This observation challenges the simplistic assumption that PDE5 inhibition is universally antiplatelet. Instead, sildenafil appears to disrupt adenosine-cAMP pathways in platelets. The precise molecular explanation remains unclear. Hypotheses include:
- Cross-talk between cGMP and cAMP signaling: Elevation of cGMP by sildenafil might somehow dampen cAMP-mediated inhibition.
- Differential compartmentalization of cyclic nucleotides: PDE5 inhibition may alter localized pools of second messengers in platelets, reshaping responsiveness.
- Unrecognized off-target effects of sildenafil: Beyond PDE5, subtle actions on other PDE isoforms could influence signaling balance.
Regardless of mechanism, the message is clinically important: sildenafil might diminish endogenous platelet inhibition by adenosine, potentially shifting thrombotic risk in certain contexts.
Clinical Implications
1. Reassurance in Stable Coronary Disease
The results support the growing consensus that sildenafil is safe in patients with stable coronary artery disease not on nitrates. By failing to exacerbate ischemia in hibernating myocardium models, sildenafil alleviates fears of coronary steal.
2. Caution in Unstable Angina
For patients with recent plaque rupture or ongoing thrombotic activity, the picture is murkier. If sildenafil blunts adenosine’s platelet-inhibitory action, it may theoretically hinder natural protective mechanisms against thrombosis. While this does not equate to overt harm in the study, it invites prudence in unstable patients.
3. Relevance to Antiplatelet Therapy
Could sildenafil attenuate the efficacy of purinergic antiplatelet drugs such as ticlopidine, clopidogrel, or dipyridamole? This remains an open question but is biologically plausible. For patients receiving such agents, drug–drug interactions at the platelet signaling level deserve careful evaluation.
4. Hemodynamic Considerations
Transient hypotension is well-documented with sildenafil, usually without lasting harm. However, in individuals with borderline perfusion pressures, these dips could matter. Physicians must remain vigilant, especially when other vasodilators are involved.
Broader Perspective: Sildenafil Beyond Erectile Dysfunction
This study highlights an important truth about modern pharmacology: drugs designed for one purpose often have far-reaching biological effects. Sildenafil has been investigated for pulmonary hypertension, heart failure with preserved ejection fraction, and even altitude sickness. Its influence on coronary physiology underscores the delicate balance between therapeutic benefit and unintended consequences.
Indeed, the story of sildenafil is one of serendipity and complexity. That a drug initially tested for angina would find fame as a treatment for erectile dysfunction, while still stirring debates about its cardiovascular safety, is a testament to the unpredictable interplay of pharmacology and clinical medicine.
Future Directions
The canine experiments by Przyklenk and Kloner provide foundational reassurance but also raise new questions:
- Do the platelet effects observed in dogs translate to humans? Human platelet biology, while similar, is not identical. Clinical studies are required to clarify risk.
- Could chronic sildenafil use modulate thrombotic risk? The study was short-term; long-term implications remain speculative.
- What are the molecular underpinnings of the sildenafil–adenosine interaction? Dissecting cGMP–cAMP cross-talk may reveal new therapeutic targets or hazards.
- How does sildenafil interact with contemporary antiplatelet drugs? With the widespread use of dual antiplatelet therapy, this is not a trivial matter.
Answering these questions is essential if sildenafil is to be used confidently in populations with significant coronary artery disease.
Conclusion
The canine studies demonstrate that sildenafil does not exacerbate myocardial ischemia in the setting of coronary stenosis. In stable hypoperfusion, its hemodynamic footprint is modest and transient. In recurrent thrombosis, however, sildenafil reveals a curious twist: by interfering with adenosine’s platelet-inhibitory action, it may blunt natural antithrombotic defenses.
For clinicians, the practical message is clear: sildenafil is generally safe in stable patients but warrants caution in unstable or high-risk cardiovascular settings. For scientists, the findings illuminate a fascinating frontier where cyclic nucleotide signaling, platelet biology, and pharmacotherapy converge.
In the end, sildenafil embodies both the promise and the perils of modern medicine—effective, transformative, and occasionally surprising.
FAQ
1. Is sildenafil safe for patients with stable coronary artery disease?
Yes. Evidence, including this study, indicates that sildenafil does not worsen perfusion or exacerbate ischemia in stable coronary disease, provided patients are not taking nitrates.
2. Why should sildenafil be used cautiously in unstable angina?
In models of recurrent thrombosis, sildenafil blunted the natural platelet-inhibitory effects of adenosine. While it did not worsen perfusion, this suggests potential interference with protective antithrombotic mechanisms.
3. Could sildenafil interact with antiplatelet medications?
Possibly. The observed platelet refractoriness to adenosine raises questions about sildenafil’s interaction with drugs targeting purinergic pathways. Clinical studies are needed to confirm or refute such concerns.