Introduction
Few challenges in reproductive medicine are as persistently frustrating as poor endometrial development following intrauterine adhesions (IUA). Despite successful surgical resection and uterine cavity restoration, many women continue to face a thin, poorly vascularized endometrium—an environment hostile to implantation and pregnancy. The thin endometrium, defined conventionally as less than 7 mm on the day of embryo transfer, remains one of the most stubborn causes of implantation failure even in otherwise optimized assisted reproductive technology (ART) cycles.
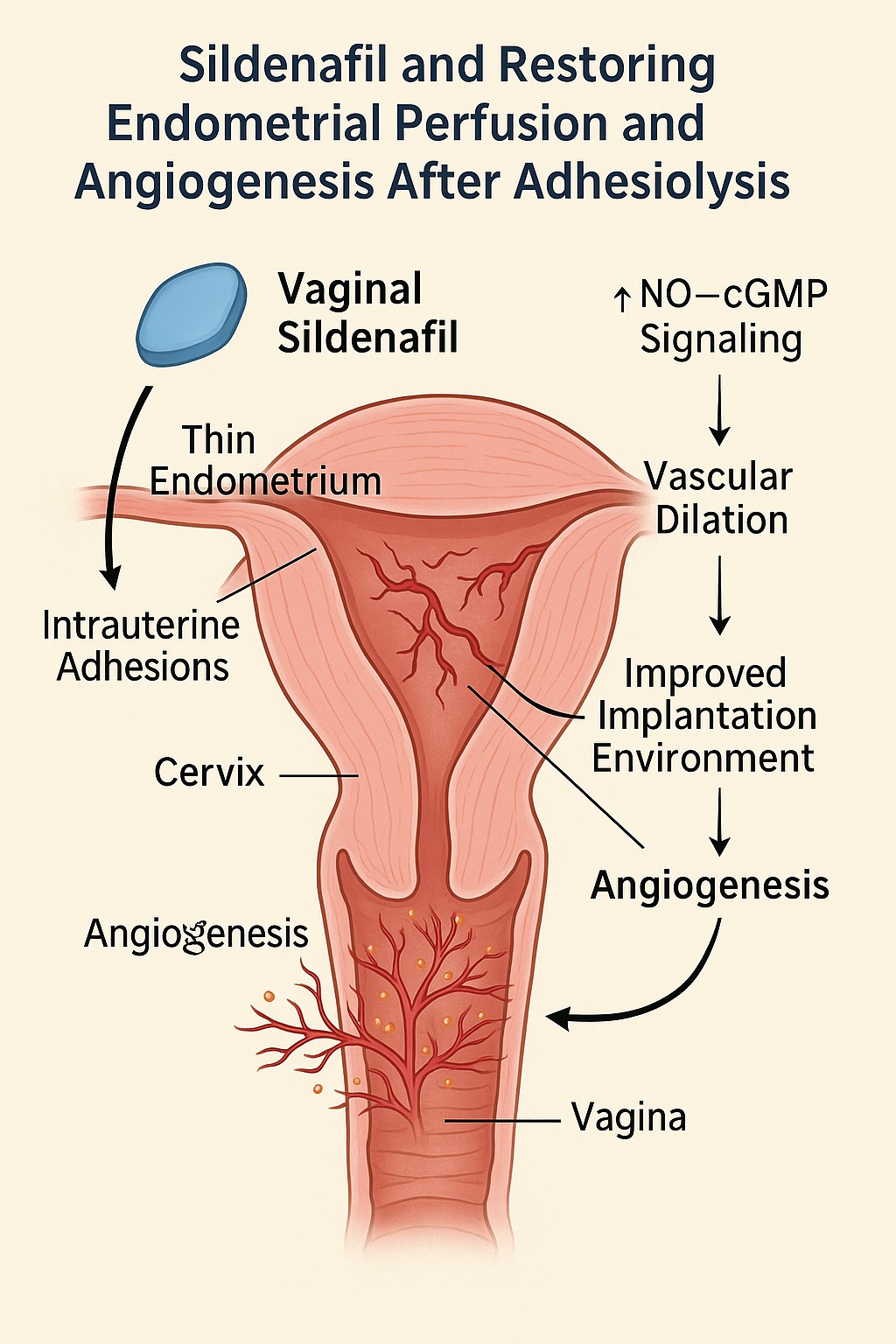
Among the pharmacologic agents investigated for endometrial rescue, sildenafil citrate—famed for its role in treating male erectile dysfunction—has emerged as a fascinating candidate. Through inhibition of phosphodiesterase type 5 (PDE5), sildenafil enhances nitric oxide (NO)–cyclic guanosine monophosphate (cGMP) signaling, a pathway that promotes vasodilation, angiogenesis, and improved tissue perfusion. The uterus, much like the corpus cavernosum, relies heavily on NO-mediated vascular relaxation for endometrial proliferation and receptivity.
The retrospective clinical study by Li et al. (2021) explored precisely this concept: whether vaginal sildenafil citrate could improve endometrial development and pregnancy outcomes in women undergoing frozen-thawed embryo transfer (FET) after adhesiolysis. The findings—though measured in scope—offer important translational clues that could refine how clinicians approach uterine rehabilitation after mechanical or inflammatory injury.
Understanding the Problem: Endometrial Failure after Adhesiolysis
Intrauterine adhesions (IUAs), or Asherman’s syndrome, result from endometrial trauma, typically following curettage, infection, or obstetric complications. Fibrosis bridges the uterine walls, compromising both the anatomical cavity and functional endometrium. Surgical resection (adhesiolysis) remains the cornerstone of management, often followed by hormonal therapy and mechanical separation (e.g., intrauterine balloons or IUDs) to prevent re-adhesion.
Yet, even when the cavity is successfully restored, the regenerative potential of the endometrium can remain blunted. Post-surgical hypoperfusion, inflammation, and loss of basal layer stem cells leave the endometrium unable to respond adequately to estrogen. The result is a thin, unresponsive lining that undermines implantation despite repeated hormone replacement therapy (HRT) cycles.
This scenario calls for an intervention that does more than stimulate proliferation—it must reawaken perfusion and oxygen delivery, reestablishing the vascular architecture that underpins receptivity. Sildenafil, with its established role in enhancing uterine blood flow, thus presents a mechanistically logical addition to post-adhesiolysis management.
The Study at a Glance: Sildenafil’s Clinical Evaluation
Li and colleagues conducted a retrospective analysis of 151 women with poor endometrial development following adhesiolysis who subsequently underwent frozen-thawed embryo transfer (FET). The cohort was divided into two groups:
- Sildenafil group (n=71): received 25 mg of vaginal sildenafil citrate four times daily during endometrial preparation.
- Control group (n=80): underwent conventional hormone replacement therapy without sildenafil.
Key outcomes assessed included endometrial thickness, blood flow, implantation rate, clinical pregnancy rate, miscarriage rate, and live birth rate.
Core Findings
- Endometrial thickness on the day of progesterone administration was significantly greater in the sildenafil group compared with controls.
- Endometrial blood flow, assessed by Doppler parameters (uterine artery pulsatility and resistance indices), improved notably with sildenafil therapy.
- Clinical pregnancy and live birth rates were higher in the sildenafil group, though the difference did not reach statistical significance—likely due to the limited sample size.
- Importantly, no adverse reactions or systemic side effects were reported, affirming the safety and tolerability of vaginal sildenafil administration.
These results suggest that enhancing local perfusion may facilitate partial recovery of endometrial structure and function, providing a more receptive substrate for embryo implantation. While modest, these improvements align with sildenafil’s pharmacodynamic profile and point to a valuable adjunct role rather than a standalone cure.
Mechanisms in Motion: How Sildenafil Restores Endometrial Function
The endometrium is a dynamic tissue governed by a delicate balance of hormonal, vascular, and cellular interactions. Successful implantation depends on a synchronized sequence—angiogenesis, stromal edema, and secretory transformation—all tightly regulated by estrogen, progesterone, and local vascular mediators.
Sildenafil acts primarily through PDE5 inhibition, which increases cGMP availability and enhances the action of NO on vascular smooth muscle. The downstream effects are multifaceted:
- Enhanced Blood Flow:
By relaxing uterine arteries and microvessels, sildenafil improves oxygen and nutrient delivery to the proliferating endometrium. - Angiogenesis and Tissue Repair:
NO and cGMP signaling stimulate vascular endothelial growth factor (VEGF) expression, promoting new vessel formation—essential for post-surgical tissue regeneration. - Anti-inflammatory and Antioxidant Effects:
Sildenafil indirectly reduces oxidative stress and inflammatory cytokine production, mitigating fibrosis and supporting stromal cell survival. - Endothelial Function Restoration:
Chronic hypoperfusion damages endothelial integrity; sildenafil helps restore endothelial nitric oxide synthase (eNOS) activity, reversing endothelial dysfunction.
These mechanisms together may convert a fibrotic, avascular endometrium into a perfused, hormonally responsive tissue, particularly valuable after adhesiolysis when baseline perfusion is compromised.
Translational Insights: Sildenafil as a Uterine Perfusion Adjuvant
From a translational standpoint, the Li et al. study reframes sildenafil not as a fertility drug per se, but as a microvascular therapeutic—an adjunct that optimizes the uterine environment for downstream hormonal and regenerative interventions.
Following adhesiolysis, clinicians often face a “biological bottleneck”: despite mechanical cavity restoration, the endometrium fails to regenerate adequately. Here, sildenafil could serve as a bridge therapy, improving local hemodynamics so that estrogen therapy, stem cell therapy, or platelet-rich plasma (PRP) applications can achieve their full effect.
Moreover, sildenafil’s vaginal route of administration deserves attention. This delivery bypasses hepatic first-pass metabolism, allowing direct uterine targeting via the “first uterine pass effect.” The result is high local concentration with minimal systemic exposure—a pharmacokinetic advantage in reproductive medicine, where safety and tolerability are paramount.
Clinically, these findings suggest sildenafil could be strategically deployed:
- Post-adhesiolysis, to enhance microvascular recovery before FET.
- In estrogen-unresponsive endometrium, as a perfusion enhancer during HRT.
- As combination therapy, alongside anti-fibrotic or angiogenic agents in complex endometrial pathologies.
Comparing Evidence: Sildenafil across Endometrial Contexts
The data from Li et al. complement a growing, though heterogeneous, body of research examining sildenafil’s role in thin endometrium management. Studies across IVF and natural-cycle protocols have yielded mixed outcomes, largely reflecting differences in patient pathology, dosing regimen, and baseline vascular function.
- In women with idiopathic thin endometrium, sildenafil modestly increased endometrial thickness and flow but inconsistently improved pregnancy outcomes.
- In patients with prior uterine trauma or adhesions, as in Li et al., the response appears more favorable—suggesting a perfusion-dependent subset that benefits most from sildenafil.
- Conversely, in cases of extensive fibrosis or basal layer loss, sildenafil alone is insufficient, as no viable stromal template remains for regeneration.
The takeaway is clear: sildenafil’s success depends less on its pharmacology and more on the biological receptivity of the endometrium to vascular rescue. It is a facilitator, not a creator, of receptivity—a nuance crucial for clinical translation.
Beyond Perfusion: Sildenafil and Molecular Receptivity
Emerging data hint that sildenafil may also influence molecular markers of endometrial receptivity, not merely its thickness. Studies have documented upregulation of VEGF, integrins (αvβ3), and leukemia inhibitory factor (LIF)—key molecules in implantation signaling. These effects, though secondary to vascular improvement, may enhance the biochemical readiness of the endometrium to accept an embryo.
Furthermore, sildenafil’s anti-fibrotic potential—through modulation of transforming growth factor-beta (TGF-β) pathways—may prevent re-adhesion after surgery, stabilizing post-adhesiolysis healing. If confirmed, this dual action (hemodynamic and anti-fibrotic) could make sildenafil a valuable component of comprehensive post-surgical uterine rehabilitation protocols.
In this light, the drug’s role extends beyond a “blood flow enhancer” to that of a molecular modulator of uterine recovery—a repositioning that aligns well with the modern paradigm of pharmacologic endometrial regeneration.
Limitations and Future Directions
While the Li et al. study provides promising evidence, several caveats temper immediate clinical adoption. Retrospective design, small sample size, and variability in adhesion severity limit the generalizability of findings. Furthermore, endometrial response to sildenafil likely depends on microvascular integrity, hormonal milieu, and prior surgical outcome, variables not uniformly controlled in retrospective analysis.
To validate sildenafil’s role, future research should include:
- Randomized controlled trials (RCTs) comparing vaginal sildenafil plus HRT versus HRT alone, with live birth as the primary endpoint.
- Perfusion imaging studies (e.g., contrast-enhanced ultrasound or MRI) to quantify hemodynamic improvement.
- Molecular assays evaluating shifts in angiogenic and receptivity markers.
- Combination therapy trials, pairing sildenafil with PRP, stem cells, or growth factors for refractory cases.
Such data would refine patient selection and establish sildenafil’s place within precision reproductive medicine, where treatment is tailored not by anatomy alone, but by vascular and molecular phenotype.
Clinical Translation: From Mechanism to Practice
Translating these findings into clinical practice requires a measured, individualized approach. Sildenafil should not be viewed as a universal solution for thin endometrium but rather as a targeted adjunct for specific scenarios:
- Post-adhesiolysis patients with demonstrable perfusion deficits.
- Estrogen-resistant endometrium in which hormonal signaling is intact but vascular response is impaired.
- Recurrent implantation failure (RIF) where uterine artery Doppler studies show high resistance indices.
In these contexts, vaginal sildenafil could be administered during the proliferative phase of the HRT cycle—typically 25 mg four times daily until progesterone initiation—while monitoring endometrial growth and perfusion via Doppler ultrasound.
Its combination with estradiol, antioxidants, and low-dose aspirin may further enhance endothelial response, although such regimens require careful titration to avoid adverse interactions.
The key is selective application within a multi-modal therapeutic strategy—where sildenafil amplifies, but does not replace, the foundational pillars of endometrial repair: estrogenic stimulation, tissue regeneration, and fibrosis control.
Conclusion
The evolving evidence, exemplified by Li et al. (2021), reframes sildenafil citrate from a symbol of male virility to a potential agent of uterine rejuvenation. By enhancing local blood flow, modulating angiogenesis, and supporting post-surgical healing, sildenafil offers a plausible pharmacologic bridge between endometrial injury and recovery.
Its clinical promise lies not in universal efficacy but in precision application—targeting women whose endometrial dysfunction stems from vascular insufficiency rather than structural loss. For these patients, vaginal sildenafil may serve as a valuable adjunct to hormone therapy, regenerative medicine, and FET preparation.
Ultimately, sildenafil’s journey from the corpus cavernosum to the uterine cavity underscores a broader truth in translational medicine: mechanisms transcend organs. When harnessed judiciously, drugs designed for one domain may breathe new life—quite literally—into another.
FAQ: Sildenafil and Endometrial Recovery
1. Can vaginal sildenafil help all women with thin endometrium?
Not necessarily. Sildenafil is most effective when poor endometrial growth results from reduced uterine blood flow rather than extensive fibrosis or hormonal deficiency. Its use should be guided by Doppler or imaging evidence of perfusion impairment.
2. How safe is vaginal sildenafil in fertility treatment?
Clinical data show excellent tolerability, with minimal systemic absorption and no adverse effects on embryos or pregnancy outcomes. Local administration avoids the cardiovascular side effects seen with oral dosing.
3. Could sildenafil be combined with other uterine regenerative therapies?
Yes, emerging strategies pair sildenafil with estrogen, platelet-rich plasma (PRP), or stem cell therapy to synergize vascular and regenerative effects. This integrative approach may define the next frontier in endometrial rehabilitation.