Introduction
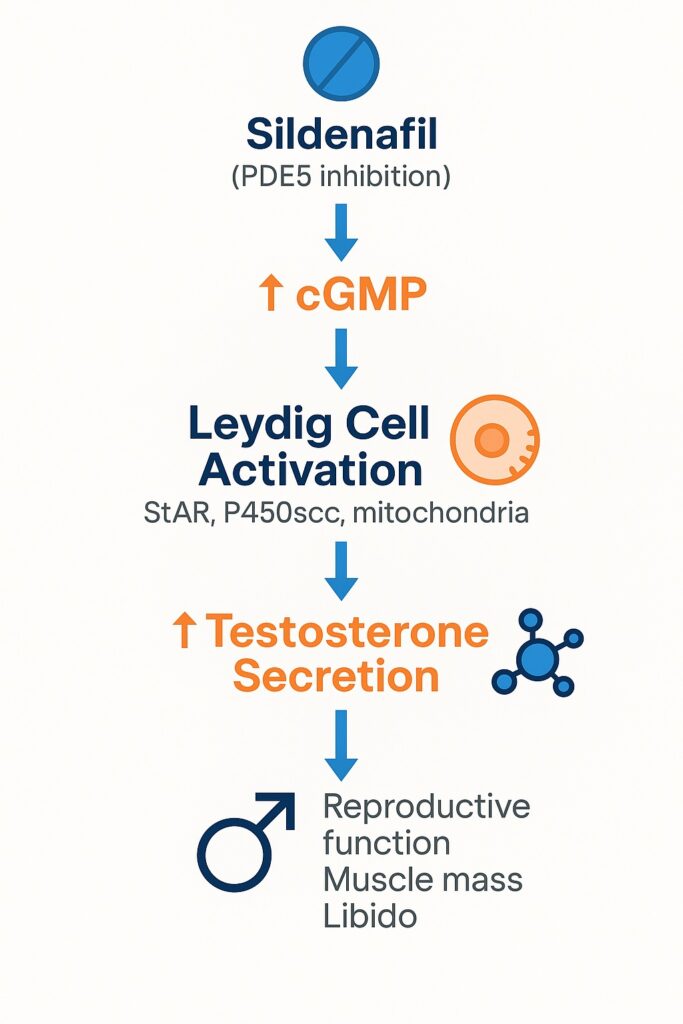
When sildenafil citrate—originally developed to treat angina—emerged as the world’s first oral therapy for erectile dysfunction, few anticipated its later identity as a pharmacological polymath. Beyond penile vascular physiology and pulmonary hypertension, sildenafil is increasingly recognized for its influence on endocrine regulation. Evidence now suggests that this PDE5 inhibitor directly stimulates steroidogenesis in Leydig cells, thereby enhancing endogenous testosterone secretion.
The implications of this finding extend far beyond urology. Testosterone is a cornerstone of male physiology, influencing reproductive function, muscle mass, mood, cognition, and cardiovascular health. Any drug capable of modifying its secretion merits serious consideration—not only for potential benefits but also for possible risks.
This article explores how chronic sildenafil treatment affects Leydig cell biology, the mechanisms behind PDE5 inhibition in steroidogenesis, and the broader implications for clinical medicine.
The cGMP Pathway and PDE5: From Vessels to Testes
At the heart of sildenafil’s pharmacology lies the NO–cGMP pathway. Nitric oxide activates soluble guanylyl cyclase, producing cyclic guanosine monophosphate (cGMP), which relaxes smooth muscle through calcium modulation. PDE5, abundantly expressed in vascular tissue, terminates this signal by degrading cGMP.
By blocking PDE5, sildenafil amplifies and prolongs cGMP action. This underpins its therapeutic role in erectile dysfunction and pulmonary hypertension. But PDE5 is not confined to blood vessels. Immunohistochemical studies have localized PDE5 within Leydig cells and peritubular myoid cells of the testis, opening the possibility that PDE5 inhibitors could directly modulate testicular physiology.
This realization reframes sildenafil not just as a vasodilator but as a potential regulator of endocrine function—a role previously underestimated in clinical practice.
Leydig Cells: The Testosterone Factories
Leydig cells, situated in the interstitial space of the testes, are the body’s primary source of testosterone. Their activity is traditionally understood as being regulated by luteinizing hormone (LH), which activates adenylate cyclase, increases cAMP, and drives steroidogenesis.
However, Leydig cells also express enzymes and receptors that integrate alternative signaling pathways, including cGMP-dependent cascades. Ultrastructural hallmarks of steroidogenic activity include abundant smooth endoplasmic reticulum (SER), mitochondria with tubular cristae, lipid droplets storing cholesterol, and specialized proteins such as steroidogenic acute regulatory (StAR) protein and cytochrome P450 side-chain cleavage enzyme (P450scc).
These molecular and morphological features position Leydig cells as both hormone factories and responsive sensors to cyclic nucleotide fluctuations. Sildenafil, by altering intracellular cGMP balance, appears to rewire their activity toward heightened testosterone synthesis.
Experimental Evidence: Sildenafil Stimulates Testosterone Production
A pivotal experimental study in mice administered sildenafil chronically at 25 mg/kg for four weeks. Leydig cells were then isolated, analyzed under electron microscopy, and subjected to immunocytochemical assays. Serum testosterone was measured by radioimmunoassay.
Key findings included:
- Ultrastructural activation: Sildenafil-treated Leydig cells displayed vesicular SER, enlarged mitochondria with disrupted cristae, and numerous vacuoles—classic features of cells engaged in active steroidogenesis.
- Enhanced steroidogenic markers: Immunolabelling for StAR, P450scc, and testosterone was markedly stronger in sildenafil-treated cells compared with controls.
- Serum testosterone elevation: Circulating testosterone levels were significantly higher in treated animals, with mean concentrations rising more than ten-fold compared to controls.
These results converge on one conclusion: sildenafil stimulates Leydig cell steroidogenesis, not merely by indirect systemic effects but through a direct influence on testicular biochemistry.
Mechanisms of Action: cGMP and Steroidogenesis
How does PDE5 inhibition translate into testosterone synthesis? Several interconnected mechanisms have been proposed:
- cGMP accumulation: By preventing PDE5-mediated degradation, sildenafil raises intracellular cGMP, which can activate protein kinase G (PKG). PKG phosphorylates targets such as StAR, enhancing cholesterol transport into mitochondria—the rate-limiting step of steroidogenesis.
- Synergy with gonadotropins: Human chorionic gonadotropin (hCG) stimulates guanylyl cyclase, generating cGMP. Sildenafil amplifies this by preventing degradation, resulting in additive effects on testosterone synthesis.
- Membranous whorles and vesicle trafficking: Structural changes in Leydig cells suggest that sildenafil enhances cholesterol mobilization and steroid precursor transport within the cell, facilitating hormone biosynthesis.
- Indirect modulation via natriuretic peptides: These peptides also elevate cGMP, and when combined with sildenafil, the effect on testosterone production may be synergistic.
Collectively, these pathways converge on a central theme: cGMP is not only a mediator of vascular relaxation but also a potent regulator of testicular steroidogenesis.
Clinical Implications: Benefits and Risks
The prospect that sildenafil elevates testosterone has several potential consequences:
- Management of hypogonadism: If confirmed in humans, sildenafil could support testosterone-deficient men by stimulating endogenous production. This might offer an alternative or adjunct to traditional testosterone replacement therapy.
- Fertility considerations: Enhanced testosterone may improve spermatogenesis and libido, though paradoxically, excessive PDE5 inhibition could also perturb sperm function.
- Pulmonary hypertension therapy: Given that high-dose sildenafil is widely used in chronic pulmonary hypertension, clinicians must be alert to unintended endocrine effects, particularly in younger patients.
Yet caution is warranted. Elevated testosterone can exacerbate conditions such as prostate hyperplasia, prostate cancer, or cardiovascular risk. Thus, the hormonal impact of long-term sildenafil must be carefully weighed in patient populations where androgen balance is clinically relevant.
Broader Endocrine Perspectives
The endocrine effects of PDE5 inhibitors extend beyond testosterone. Research suggests that sildenafil modulates adrenal steroidogenesis, influences cortisol regulation, and interacts with metabolic pathways. These findings challenge the outdated notion of PDE5 inhibitors as strictly “vascular drugs.”
Moreover, the dual role of testosterone itself—vasodilator in vascular beds and regulator of reproductive tissues—suggests that sildenafil’s endocrine effects could contribute indirectly to its vascular benefits. Enhanced testosterone may potentiate endothelial nitric oxide synthase activity, further reinforcing vasodilation.
This duality raises the intriguing possibility that part of sildenafil’s efficacy in pulmonary hypertension may derive from endocrine as well as vascular mechanisms.
Future Directions
Several lines of investigation remain open:
- Human studies: While animal data are compelling, robust human trials are necessary to confirm whether chronic sildenafil elevates testosterone significantly and consistently.
- Isoform selectivity: PDE5 expression in Leydig cells offers a direct mechanism, but other PDE isoforms may also contribute to steroidogenesis. Targeting these selectively could refine pharmacological control.
- Therapeutic exploitation: Could sildenafil or related agents be deliberately used to stimulate endogenous testosterone in hypogonadal men? If so, what are the long-term consequences compared to standard hormone therapy?
- Safety balance: How do we reconcile potential androgen benefits with risks of prostate pathology, erythrocytosis, or cardiovascular strain? These questions demand careful longitudinal studies.
Conclusion
The discovery that sildenafil stimulates testosterone secretion by activating Leydig cell steroidogenesis reframes our understanding of this iconic drug. What began as a vascular therapy for erectile dysfunction and pulmonary hypertension now reveals itself as an unanticipated modulator of endocrine function.
Chronic PDE5 inhibition increases cGMP in Leydig cells, enhances steroidogenic protein activity, remodels cellular ultrastructure, and ultimately elevates serum testosterone. These findings illuminate an unexpected intersection between vascular pharmacology and reproductive endocrinology.
Clinicians should be aware that the widespread use of sildenafil—whether for sexual dysfunction or pulmonary hypertension—may carry endocrine consequences. For some patients, this represents an added therapeutic benefit; for others, a potential risk. As always in medicine, context and careful monitoring will determine whether this pharmacological twist of fate proves boon or burden.
FAQ
1. Does sildenafil increase testosterone levels in men?
Animal studies strongly suggest that chronic sildenafil elevates testosterone via Leydig cell stimulation. Preliminary human data also support this, but more robust clinical trials are needed to confirm consistency and safety.
2. How does sildenafil stimulate testosterone production?
By inhibiting PDE5, sildenafil increases intracellular cGMP, which activates protein kinase G. This enhances steroidogenic acute regulatory protein activity and P450scc function, boosting testosterone synthesis in Leydig cells.
3. Should sildenafil be considered a treatment for hypogonadism?
Not yet. While findings are promising, sildenafil is not approved as a therapy for low testosterone. Its use remains primarily for erectile dysfunction and pulmonary hypertension, though future research may broaden its indications.