Introduction
Benign prostatic hyperplasia (BPH) is an almost inevitable guest in the lives of aging men. By the age of 80, up to 80% of men will show histological evidence of prostate enlargement, often accompanied by bothersome lower urinary tract symptoms (LUTS). These symptoms—frequent urination, weak urinary stream, incomplete bladder emptying—may seem trivial at first but can progress to bladder dysfunction, recurrent urinary infections, or even renal failure. In parallel, erectile dysfunction (ED) often emerges, creating a double burden on quality of life. Traditionally, physicians approached these conditions separately, one with α1-adrenoreceptor antagonists or 5α-reductase inhibitors, the other with phosphodiesterase-5 inhibitors (PDE5Is) such as sildenafil. But what if the same drug could address both?
The research underpinning this discussion stems from a controlled rabbit model of testosterone-induced prostate hypertrophy. Investigators explored whether sildenafil citrate, a widely prescribed PDE5I, could reduce prostate enlargement and simultaneously improve bladder function. Their findings suggest that sildenafil may indeed offer a dual therapeutic benefit: antiproliferative effects on the prostate and smooth muscle relaxation in the lower urinary tract.
This article unpacks the science, contextualizes the findings, and considers how sildenafil may reshape future management strategies for BPH and associated urinary tract complications.
The Burden of Benign Prostatic Hyperplasia
BPH is far from a rare curiosity—it is the rule rather than the exception in male aging. Autopsy studies reveal a prevalence of around 8% in men in their forties, 50% in their sixties, and nearly 80% by the ninth decade of life. Such numbers alone would make BPH a major medical concern. But more pressing is its impact on quality of life and health.
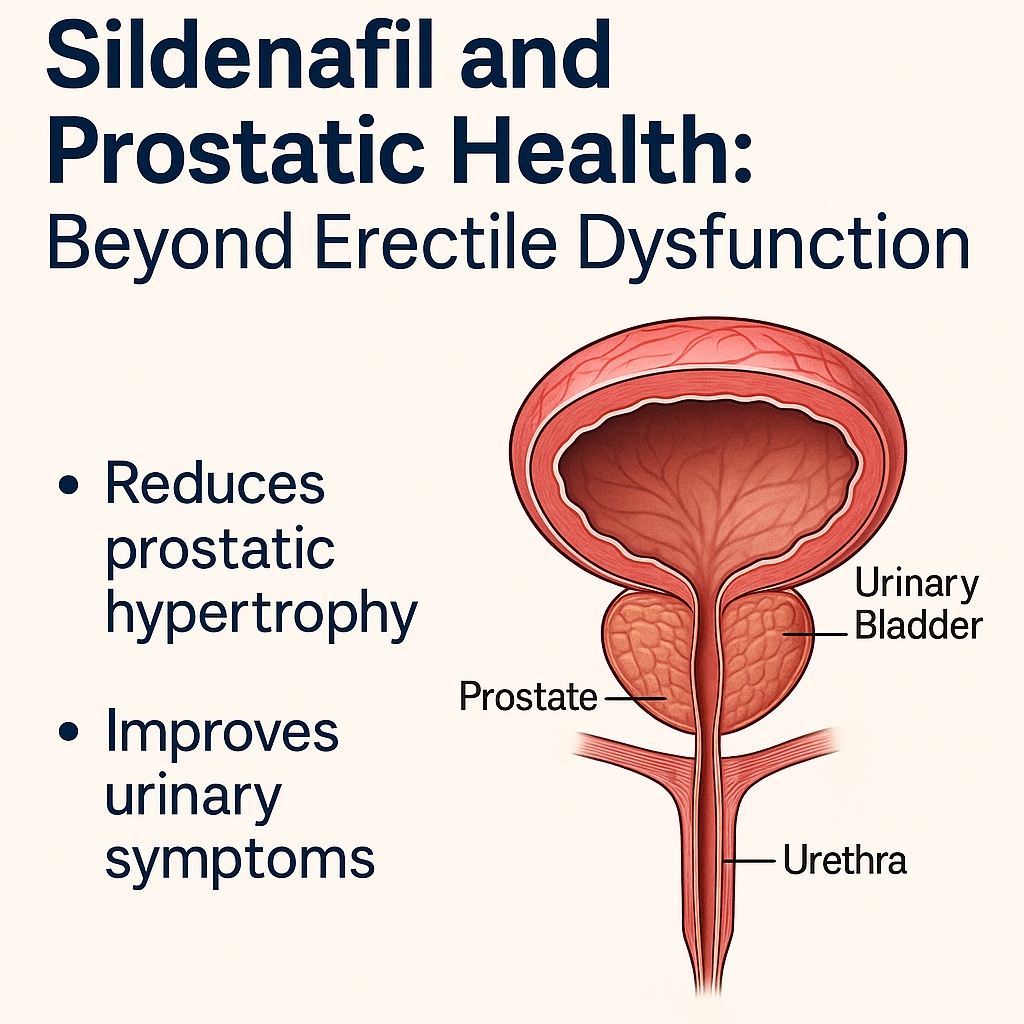
As the prostate enlarges, it compresses the urethra and alters bladder physiology. The bladder wall hypertrophies in an attempt to compensate for outflow resistance, leading to irritative symptoms. Over time, this compensation fails, resulting in incomplete emptying, high residual urine volumes, infections, stone formation, and, in advanced stages, hydronephrosis and renal impairment. Patients report nocturia, urgency, and diminished urinary flow—symptoms that disrupt sleep, productivity, and psychological well-being.
Current therapeutic strategies target two primary pathways: α1-adrenoreceptor antagonists relax smooth muscle at the bladder neck and prostate, while 5α-reductase inhibitors reduce dihydrotestosterone-driven prostatic growth. Both drug classes have undeniable efficacy, but they are not without drawbacks. α1-blockers may cause hypotension and dizziness, while 5α-reductase inhibitors frequently impair libido and sexual performance. Against this backdrop, the search for new therapeutic approaches is not a luxury but a necessity.
Sildenafil: From Penile Smooth Muscle to the Prostate
Sildenafil (Viagra®) is firmly etched in the public consciousness as the quintessential ED drug. Approved by the FDA in 1998, it rapidly became one of the most prescribed medications worldwide. Its mechanism is elegant: sexual stimulation triggers nitric oxide (NO) release, which activates guanylyl cyclase, producing cyclic guanosine monophosphate (cGMP). Elevated cGMP lowers intracellular calcium, relaxing smooth muscle and enhancing blood flow to the corpus cavernosum, producing erection.
cGMP, however, is fleeting. Phosphodiesterase type 5 (PDE5) rapidly degrades it, curtailing the erection. By blocking PDE5, sildenafil preserves cGMP and prolongs vasodilation. What was once considered a penile-specific process is now recognized as a more general smooth muscle relaxation pathway extending into the prostate, bladder, and urethra.
Studies have identified PDE5 expression in the prostate and bladder neck, regions directly implicated in LUTS. Moreover, impaired NO/cGMP signaling has been implicated in prostate pathology. These findings raise the possibility that sildenafil might simultaneously alleviate LUTS and exert disease-modifying effects on prostatic growth.
A Rabbit Model of Prostate Hypertrophy
Animal models are essential for probing complex diseases in controlled conditions. In this study, male New Zealand white rabbits served as the subjects. Prostatic hypertrophy was induced by administering testosterone propionate for eight weeks, mimicking androgen-driven prostatic overgrowth observed in aging men.
The design included three groups:
- Control group: untreated rabbits.
- Testosterone group: rabbits receiving testosterone alone.
- Testosterone + Sildenafil group: rabbits first exposed to testosterone, then treated with sildenafil (5 mg/kg/day) for eight weeks.
This design allowed researchers to compare baseline physiology, hypertrophy-induced pathology, and the therapeutic impact of sildenafil. At study completion, prostates were excised, weighed, and examined histologically. In parallel, strips of bladder neck muscle were subjected to organ bath experiments to test contractility and relaxation in response to phenylephrine and sildenafil.
The approach provided not only structural evidence (size, weight, histology) but also functional insight into how sildenafil modulates bladder smooth muscle tone.
Impact of Sildenafil on Prostate Size
Testosterone administration predictably enlarged the rabbit prostates, increasing their mean weight by more than 60% compared with controls. Hypertrophy was confirmed histologically, with papillary projections, expanded acinar structures, and thickened epithelial linings—hallmarks of BPH.
Sildenafil treatment reversed much of this pathology. After eight weeks of therapy, prostate weights dropped by more than one-third compared to hypertrophied controls, approaching near-normal values. Importantly, the body/prostate weight ratio not only normalized but exceeded control values, suggesting not merely stabilization but genuine regression of hypertrophic changes.
Histology told the same story in finer detail. Sildenafil-treated prostates showed reduced papillary projections, thinner epithelium, and smaller acinar areas. The tissue architecture resembled that of the healthy controls more than the testosterone-only group. This reversal of hyperplastic and hypertrophic changes provides strong evidence that sildenafil may act as more than a symptomatic agent—it may exert true disease-modifying effects in BPH.
Bladder Neck Relaxation: A Functional Bonus
Prostate size is one aspect of the BPH problem, but bladder outlet resistance is another. Patients care less about gland weight and more about urinary flow. Thus, the organ bath experiments testing sildenafil’s impact on bladder neck muscle are particularly illuminating.
When phenylephrine was applied, the bladder neck muscle strips contracted consistently, mimicking adrenergic-driven bladder outlet obstruction. Adding sildenafil produced a robust relaxation response, reducing tension by nearly one-third. The effect was dose-dependent, with maximal relaxation achieved at micromolar concentrations of the drug.
Mechanistic experiments confirmed the central role of the NO pathway. Blocking NO synthase with L-NAME attenuated sildenafil’s effect, while adding sodium nitroprusside, an NO donor, enhanced it. This aligns with the canonical NO/cGMP signaling mechanism by which sildenafil exerts its vasodilatory and smooth muscle-relaxant effects.
For patients, this dual mechanism—shrinking the prostate and relaxing the bladder neck—could mean a powerful synergy: reduced obstruction, improved flow, and fewer LUTS.
Anti-Inflammatory and Anti-Fibrotic Dimensions
BPH is not just about cell proliferation; inflammation and fibrosis are central to its progression. Recent studies suggest that PDE5Is, including sildenafil, may blunt pro-inflammatory cytokines such as interleukin-8 and inhibit stromal cell proliferation. By activating cGMP/PKG signaling, sildenafil interferes with tissue remodeling processes that perpetuate hyperplasia and fibrosis.
The histological improvements observed in the rabbit prostates—less epithelial thickening, fewer papillary projections—support this anti-inflammatory and anti-fibrotic hypothesis. It is not just that the prostate shrinks; it appears to remodel toward healthier tissue architecture.
If validated in clinical trials, these properties could position sildenafil as more than a symptomatic reliever. It could emerge as a preventive agent slowing BPH progression, reducing the need for surgery, and preserving bladder and kidney function in the long term.
Clinical Relevance: Where Science Meets Practice
For the practicing clinician, the idea of repurposing sildenafil for BPH is tantalizing but must be approached with rigor. The benefits are appealing:
- Dual symptom relief: simultaneously improving ED and LUTS.
- Favorable safety profile: sildenafil is generally well tolerated compared to α1-blockers and 5α-reductase inhibitors.
- Potential disease modification: reversing hypertrophy and fibrosis, not just masking symptoms.
However, caveats remain. The rabbit study is preclinical; human physiology, dosing, and comorbidities may alter outcomes. The long-term safety of daily sildenafil in older men, especially regarding cardiovascular health and fertility, needs comprehensive evaluation. Current clinical trials with PDE5Is, such as tadalafil, have shown improvements in LUTS, lending credence to this approach. Yet, large-scale, long-duration trials are essential before sildenafil can be routinely prescribed for BPH.
Nevertheless, the principle is sound: targeting NO/cGMP pathways offers a fresh pharmacological angle on BPH, one that integrates vascular biology, inflammation, and smooth muscle tone.
Future Directions
Research into PDE5Is and BPH is expanding. Key questions for future exploration include:
- Optimal dosing: What daily dose balances efficacy with safety for long-term use?
- Comparative effectiveness: How does sildenafil compare with tadalafil, vardenafil, or conventional therapies in head-to-head trials?
- Combination therapy: Could PDE5Is be safely combined with α1-blockers or 5α-reductase inhibitors for additive benefit?
- Reproductive outcomes: Does long-term PDE5I use affect sperm quality or fertility?
- Molecular targets: Can the anti-inflammatory and antifibrotic effects of sildenafil be harnessed to design next-generation agents specifically for BPH?
Answering these questions requires collaboration between basic scientists, pharmacologists, and clinicians. But the path forward is clear: the NO/cGMP axis is a promising therapeutic frontier for prostatic diseases.
Conclusion
The study of sildenafil in testosterone-induced prostatic hypertrophy provides compelling preclinical evidence that this well-known ED drug may double as a therapy for BPH. By reducing prostate weight, reversing histological hallmarks of hyperplasia, and relaxing bladder neck smooth muscle, sildenafil addresses both structural and functional aspects of urinary obstruction.
The dual benefit—improving erectile function while mitigating LUTS—makes it uniquely attractive in aging men who often suffer both conditions simultaneously. Though further human studies are essential, sildenafil and related PDE5Is may soon claim a broader role in urological practice, shifting from bedroom aid to bladder ally.
FAQ
1. Can sildenafil really shrink the prostate?
Preclinical studies, including the rabbit model discussed here, show that sildenafil can reduce prostate weight and reverse histological features of hyperplasia. While promising, clinical trials in humans are needed to confirm whether the same effect occurs in patients.
2. How does sildenafil improve urinary symptoms?
Sildenafil relaxes smooth muscle in the bladder neck and prostate by enhancing nitric oxide–cGMP signaling. This reduces outflow resistance, improves urinary flow, and may alleviate common LUTS such as weak stream and frequency.
3. Is sildenafil safer than current BPH medications?
Sildenafil is generally well tolerated, with mild side effects such as headache and flushing. Unlike α1-blockers, it rarely causes hypotension, and unlike 5α-reductase inhibitors, it does not typically impair libido. However, long-term safety in older men with comorbidities must be studied further.