Introduction
The story of sildenafil, originally introduced as a treatment for erectile dysfunction, is evolving in directions few pharmacologists anticipated. Beyond its now iconic role as a phosphodiesterase type 5 (PDE5) inhibitor for vasodilation, sildenafil has been investigated for pulmonary hypertension, neonatal cardiopulmonary disorders, and even altitude-related hypoxia. More recently, a new frontier has emerged: its potential to address metabolic disturbances during pregnancy, particularly those linked to hyperandrogenism and gestational cardiometabolic syndrome.
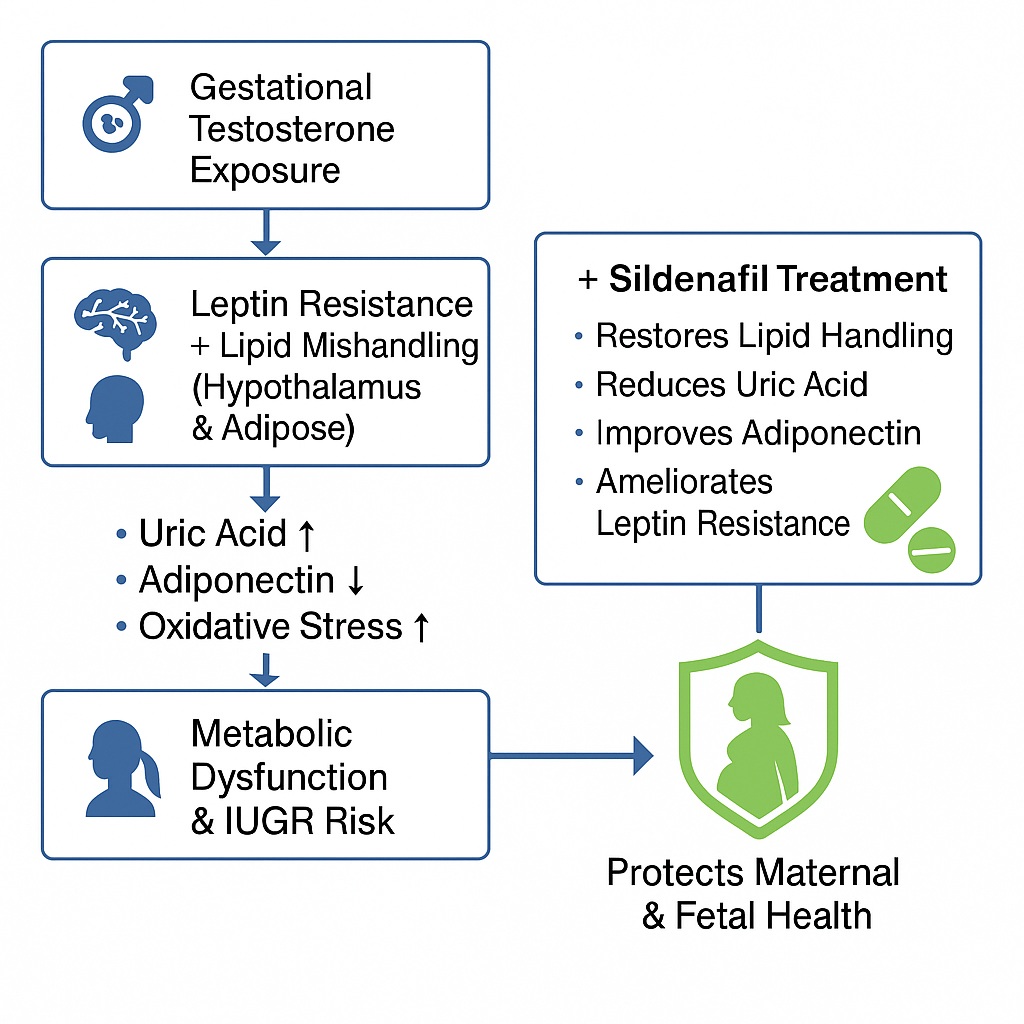
One of the central discoveries in this regard is sildenafil’s ability to ameliorate leptin resistance and normalize lipid handling within the hypothalamic-adipose axis. These findings, derived from carefully controlled experimental studies in testosterone-exposed pregnant rats, may have implications for preventing intrauterine growth restriction (IUGR) and improving maternal health outcomes.
This article unpacks the meaning of these results, explaining the physiology of leptin resistance, the disturbances caused by gestational hyperandrogenism, and how sildenafil might serve as a protective pharmacological tool. The discussion also underscores the broader significance of hypothalamic-adipose communication and metabolic regulation during pregnancy—a period when even minor biochemical shifts can resonate across maternal and fetal systems.
Leptin, Adipose Tissue, and the Hypothalamic Connection
Leptin, a 16 kDa peptide hormone secreted primarily by adipocytes, occupies a unique place in the metabolic orchestra. It conveys the state of peripheral energy stores to the brain, particularly the hypothalamus, which in turn regulates appetite, satiety, thermogenesis, and energy expenditure. In principle, elevated leptin levels should suppress food intake and protect against weight gain. In practice, however, chronic elevations of leptin in obesity are associated not with leanness but with leptin resistance—a state in which high leptin fails to produce the expected anorexic effect.
Several mechanisms have been proposed for leptin resistance: impaired leptin receptor signaling, defective transport across the blood–brain barrier, or maladaptive developmental programming of hypothalamic neurons. Regardless of the mechanism, the end result is the same: the brain fails to register satiety despite high circulating leptin, driving persistent feeding and metabolic dysfunction.
Adipose tissue signals to the hypothalamus not only via leptin but also through lipid derivatives, particularly free fatty acids (FFAs). Even subtle changes in postprandial fatty acid levels can modulate hypothalamic responses, signaling satiety or hunger depending on the context. The failure of this lipid-based communication—whether through overproduction, impaired clearance, or altered hypothalamic lipid handling—sets the stage for insulin resistance, dyslipidemia, and metabolic disease.
Gestational Hyperandrogenism and Metabolic Stress
Pregnancy imposes unique metabolic demands. Under physiological conditions, maternal lipid and glucose handling adapt to ensure adequate energy supply to the growing fetus. However, in states of gestational hyperandrogenism—such as those linked to polycystic ovary syndrome (PCOS)—this adaptation becomes distorted.
Excess maternal testosterone disrupts both lipid and glucose homeostasis, resulting in features reminiscent of metabolic syndrome: hyperinsulinemia, hypertriglyceridemia, and leptin resistance. These disturbances not only jeopardize maternal well-being but also endanger the fetus through mechanisms like intrauterine growth restriction (IUGR), preeclampsia, and impaired placentation.
Crucially, disturbances in the hypothalamic-adipose axis amplify these risks. The hypothalamus, with its appetite-regulating nuclei, becomes vulnerable to oxidative stress, uric acid accumulation, and inflammatory signaling. Meanwhile, adipose tissue exhibits enhanced lipolysis and altered secretion of adipokines (leptin and adiponectin), compounding systemic metabolic dysregulation.
This interplay is not merely academic. It defines the maternal-fetal metabolic environment and can leave lasting epigenetic imprints on offspring, predisposing them to cardiometabolic disease later in life.
The Role of Sildenafil Beyond Vasodilation
Sildenafil’s classical mechanism is well established: it inhibits PDE5, thereby preventing the degradation of cGMP and amplifying nitric oxide–mediated vasodilation. This effect explains its therapeutic use in erectile dysfunction and pulmonary arterial hypertension.
But can sildenafil influence metabolism? The answer, according to recent experimental evidence, is yes—albeit in mechanisms not strictly limited to PDE5 inhibition. In testosterone-exposed pregnant rats, sildenafil administration produced several notable effects:
- Improved body weight regulation: Rats exposed to testosterone exhibited impaired weight gain and increased adipose mass dysregulation. Sildenafil attenuated these effects, restoring a healthier phenotype.
- Amelioration of leptin resistance: By lowering the leptin-to-adiponectin ratio and improving hypothalamic adiponectin levels, sildenafil counteracted testosterone-induced leptin resistance.
- Normalization of lipid handling: Sildenafil reduced hypothalamic free fatty acids, increased adipose triglyceride storage, and lowered systemic hypertriglyceridemia, thereby rebalancing lipid communication between adipose tissue and hypothalamus.
- Reduction of uric acid burden: Elevated plasma and hypothalamic uric acid, a hallmark of metabolic stress, were reduced with sildenafil. This in turn supported adiponectin production and dampened pro-inflammatory cascades.
Interestingly, many of these protective effects occurred independently of PDE5 activity. Despite reducing PDE5 activity and altering cGMP levels, sildenafil’s metabolic influence extended to antioxidant regulation, uric acid modulation, and adipokine signaling.
Hypothalamic-Adipose Lipid Mishandling: A Central Theme
One of the study’s most striking insights is that metabolic health during pregnancy cannot be understood by focusing on one tissue alone. The hypothalamus and adipose tissue engage in a bidirectional dialogue, and when this dialogue falters, systemic pathology ensues.
Gestational testosterone exposure increased hypothalamic FFAs while reducing cholesterol accumulation, a profile indicative of lipolysis and metabolic stress. In parallel, adipose tissue displayed reduced triglycerides and altered leptin/adiponectin secretion. Together, these changes reinforced leptin resistance and insulin insensitivity.
Sildenafil interrupted this maladaptive feedback loop. By restoring triglyceride handling in adipose tissue and reducing hypothalamic FFAs, it dampened the signals that would otherwise perpetuate hyperphagia, insulin resistance, and inflammatory responses. This normalization of hypothalamic-adipose communication represents a key therapeutic axis for preventing metabolic decline during pregnancy.
The Uric Acid–Adiponectin–Inflammation Triangle
Among the biochemical parameters studied, uric acid deserves special attention. Hyperuricemia is more than a biomarker of metabolic stress; it actively suppresses adiponectin production, fosters oxidative stress, and accelerates leptin resistance. In testosterone-exposed pregnant rats, both plasma and hypothalamic uric acid were significantly elevated.
Sildenafil reduced uric acid levels, an effect linked to improved adiponectin secretion and reduced hypothalamic inflammation. Elevated adiponectin, in turn, contributed to reversing leptin resistance and restoring hypothalamic sensitivity to peripheral signals. This triangular relationship—uric acid, adiponectin, and inflammation—offers an elegant explanation for sildenafil’s broader metabolic benefits.
Antioxidant Signaling and the Nrf2 Pathway
Another layer of complexity lies in oxidative stress. The hypothalamus is exquisitely sensitive to reactive oxygen species (ROS), and gestational testosterone exposure reduced activity of the antioxidant regulator Nrf2 and its downstream enzyme catalase. The result was a hypothalamic environment skewed toward oxidative damage.
While sildenafil did not fully restore Nrf2-dependent antioxidant defenses, it partially improved hypothalamic Nrf2 levels compared with testosterone-only exposure. This partial effect suggests that sildenafil’s primary strength lies not in direct antioxidant modulation but in alleviating upstream metabolic stressors—such as lipid mishandling and uric acid excess—that generate ROS in the first place.
Clinical Implications: The Case for Maternal Protection
The translational implications of these findings are compelling. Gestational hyperandrogenism, whether arising from PCOS or other conditions, predisposes women to pregnancy complications and long-term cardiometabolic disease. Interventions that stabilize the hypothalamic-adipose axis could therefore benefit both maternal and fetal outcomes.
Sildenafil, with its established safety profile in non-pregnant adults and emerging use in obstetric research, offers a candidate worthy of further clinical exploration. Its ability to attenuate leptin resistance, correct lipid mishandling, and reduce uric acid burden may complement its vasodilatory effect at the feto-placental interface, broadening its therapeutic potential in preventing IUGR and metabolic dysfunction.
Of course, caution remains essential. Rodent findings cannot be extrapolated uncritically to humans, and fetal outcomes must be rigorously assessed in well-designed trials. Nevertheless, the possibility that a widely used drug could protect maternal metabolism during pregnancy is both scientifically exciting and clinically significant.
Conclusion
Sildenafil’s journey from a urological agent to a potential protector of maternal metabolism highlights the serendipity of pharmacology. In testosterone-exposed pregnant rats, sildenafil ameliorated leptin resistance, normalized lipid handling between adipose tissue and hypothalamus, reduced uric acid burden, and partially restored antioxidant defenses. These effects occurred largely independent of PDE5 activity, suggesting that sildenafil’s metabolic benefits extend beyond its canonical mechanism.
If validated in human studies, sildenafil may become part of a broader strategy to combat gestational metabolic disorders, protect against IUGR, and safeguard long-term maternal and offspring health. At the very least, this research expands our appreciation of the hypothalamic-adipose axis as a critical battleground in the war against metabolic disease.
FAQ
1. What is leptin resistance, and why does it matter in pregnancy?
Leptin resistance occurs when high levels of leptin fail to suppress appetite and regulate energy balance. In pregnancy, especially under hyperandrogenic conditions, leptin resistance contributes to metabolic dysfunction, insulin resistance, and adverse fetal outcomes.
2. How does sildenafil improve metabolic health in this context?
Sildenafil reduced leptin resistance, normalized lipid handling between adipose tissue and hypothalamus, and lowered uric acid levels in testosterone-exposed pregnant rats. These effects helped restore systemic energy balance and reduce inflammation.
3. Could sildenafil be used in pregnant women with PCOS or metabolic syndrome?
While the animal studies are promising, human trials are needed before recommending sildenafil in pregnancy. Its established safety in other conditions makes it an attractive candidate, but maternal-fetal safety and efficacy must be confirmed.