Introduction
When sildenafil citrate entered the medical stage in the late 1990s, its fame rested firmly on its ability to rescue erections through selective inhibition of phosphodiesterase type 5 (PDE5). Yet pharmacology often writes richer stories than the ones drug developers intend. With the passage of time, sildenafil has revealed influences far beyond penile smooth muscle relaxation. A growing body of evidence points toward testicular effects, specifically on Leydig cells, where steroidogenesis — the synthesis of testosterone — is fine-tuned by the interplay of cyclic nucleotide signaling.
The landmark study by Andrić and colleagues (2010) demonstrated that chronic sildenafil administration in vivo stimulates Leydig cell steroidogenesis. This effect is not simply an accidental artifact, but a biologically coherent outcome rooted in the cAMP and cGMP signaling cascade and the regulation of steroidogenic acute regulatory (StAR) protein.
In this article, we explore the physiological context, experimental findings, mechanistic insights, and clinical implications of sildenafil’s testicular actions, while placing them within the broader narrative of men’s reproductive endocrinology.
The Role of Leydig Cells in Male Reproductive Health
Leydig cells, located in the interstitial compartment of the testes, are the body’s primary testosterone producers. Their activity is orchestrated by luteinizing hormone (LH), which binds to the LH receptor (LHR), activating adenylyl cyclase and increasing intracellular cAMP. This, in turn, activates protein kinase A (PKA), setting off a cascade that culminates in the production of testosterone.
The linchpin of this process is the StAR protein. StAR mediates cholesterol transfer into the inner mitochondrial membrane, the rate-limiting step of steroidogenesis. Without StAR, steroid hormone production collapses, regardless of the presence of enzymes further down the biosynthetic line.
Leydig cell physiology is not solely a story of cAMP. The NO–cGMP pathway also contributes, albeit in a more nuanced fashion. Low levels of nitric oxide stimulate cGMP production and protein kinase G (PKG) activation, supporting steroidogenesis. High levels, however, inhibit steroidogenic enzymes, demonstrating the importance of balance.
Sildenafil in the Testis: Why It Matters
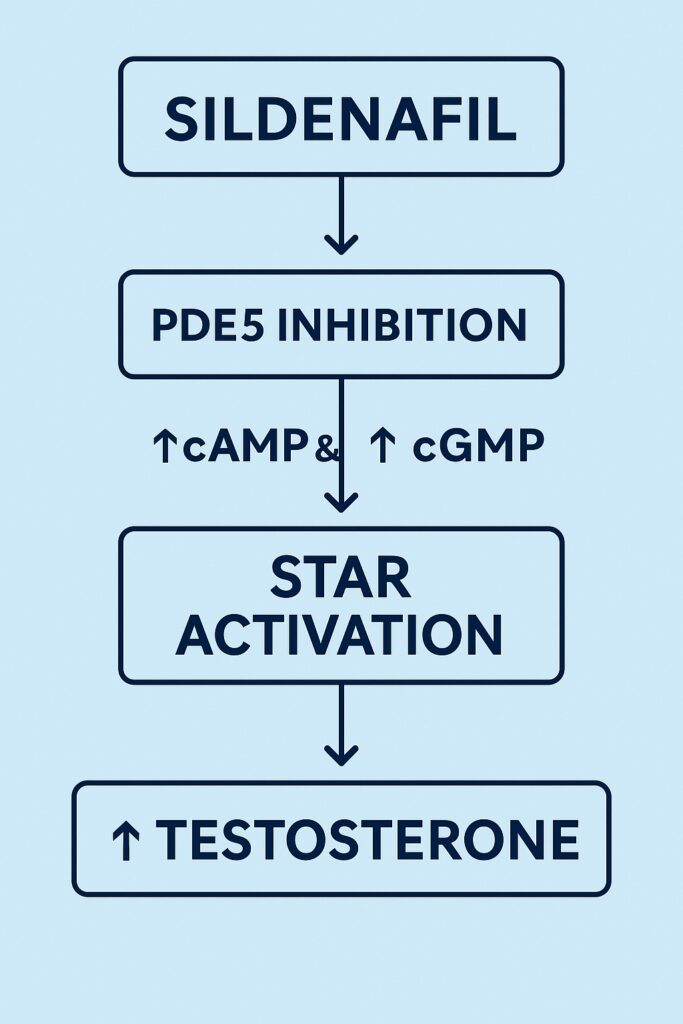
Sildenafil works by selectively inhibiting PDE5, the enzyme that hydrolyzes cGMP. PDE5 is abundantly expressed in penile corpus cavernosum, but also in vascular smooth muscle and, importantly, in Leydig cells. Thus, PDE5 inhibition within the testis has direct implications: sustained cGMP levels, altered interaction with cAMP signaling, and downstream enhancement of steroidogenesis.
Andrić et al. sought to evaluate precisely this by treating rats with sildenafil chronically and then assessing serum testosterone levels, Leydig cell responsiveness, intracellular signaling markers, and expression of steroidogenic machinery. The results expanded the conceptual boundaries of PDE5 inhibition well beyond erectile physiology.
Experimental Evidence: Sildenafil Boosts Testosterone
The experimental framework involved adult male rats treated with sildenafil at 1.25 mg/kg daily for 30 days. This dose mirrors chronic therapeutic exposure rather than acute supraphysiological intervention.
The major findings were:
- Serum testosterone increased significantly in sildenafil-treated animals compared to controls.
- Isolated Leydig cells from treated rats produced more testosterone under both basal and hCG-stimulated conditions, proving the effect was intrinsic to the cells, not merely systemic.
- cAMP levels were elevated, owing to reduced expression of phosphodiesterase-4A (PDE4A), the main cAMP-degrading isoform.
- cGMP sensitivity improved, with upregulation of guanylyl cyclase subunits (GUCY1A1, GUCY1B1) and downregulation of inducible nitric oxide synthase (NOS2), favoring physiological NO signaling.
- StAR protein was more abundant in its mature, functionally active form, supporting enhanced cholesterol transport and steroidogenic efficiency.
- Selective upregulation of CYP17A1 occurred, boosting the enzymatic machinery for androgen biosynthesis.
The convergence of these findings points to a robust, multifaceted enhancement of Leydig cell function.
Mechanistic Insights: cAMP and cGMP in Concert
The novelty of Andrić’s work lies in unraveling how sildenafil coordinates two signaling cascades that were long thought to operate in parallel but separate domains.
- On the cAMP axis: Sildenafil reduced PDE4A expression, allowing cAMP to accumulate more robustly following LH or hCG stimulation. This potentiated the canonical steroidogenic cascade, activating PKA and transcription factors for steroidogenic enzymes.
- On the cGMP axis: PDE5 inhibition directly sustained cGMP levels. More importantly, sildenafil reprogrammed Leydig cells by lowering NOS2 (reducing excessive NO that can be inhibitory) and upregulating guanylyl cyclase, making cells exquisitely sensitive to physiological NO. This resulted in PKG activation and phosphorylation of StAR.
- At the StAR checkpoint: Both cascades converge. PKA (from cAMP) and PKG (from cGMP) enhance StAR activity, ensuring efficient cholesterol transfer into mitochondria — the indispensable step in steroid hormone biosynthesis.
The outcome: more testosterone, produced more efficiently, without altering total collagen or tissue volume but by enhancing qualitative steroidogenic capacity.
Broader Biological Significance
Why does this matter? Testosterone is not just a reproductive hormone. Its roles extend to:
- Maintaining libido and erectile function.
- Supporting spermatogenesis and fertility.
- Preserving muscle mass, bone density, and metabolic health.
- Contributing to mood, energy, and cognition.
Thus, by subtly enhancing endogenous testosterone production, sildenafil could serve as more than a symptomatic drug for ED. It may act as a functional modulator of male endocrine health.
Interestingly, this could partially explain why chronic PDE5 inhibitor use in some clinical studies correlates with modest increases in serum testosterone. The rat data provide mechanistic credibility to those observations.
Clinical Implications
If translational validity holds, the clinical implications are significant:
- Adjunct in hypogonadism: Men with borderline low testosterone may benefit not only from improved erectile function but also from mild androgen enhancement through PDE5 inhibition.
- Dual therapy with TRT: Combining PDE5 inhibitors with testosterone replacement therapy may synergize, enhancing sexual outcomes in men with hypogonadism plus ED.
- Metabolic syndrome and diabetes: These populations often harbor both ED and low testosterone. Sildenafil’s endocrine effect may help address both simultaneously.
Of course, caution is necessary. Testosterone elevation is modest, not a substitute for formal replacement in men with primary testicular failure. Moreover, potential risks — prostate health, erythrocytosis, or cardiovascular impact — require careful monitoring in any clinical extension.
Limitations and Open Questions
While compelling, Andrić’s findings must be interpreted with caution:
- Rat Leydig cells, though comparable, are not identical to human Leydig cells.
- The study used one dose and duration; dose–response and long-term outcomes remain undefined.
- The impact on fertility, spermatogenesis, and broader hypothalamic–pituitary–gonadal (HPG) axis regulation is not fully explored.
- Clinical data in men remain fragmentary. Not all studies confirm testosterone increases with PDE5 inhibitors, and variability may reflect comorbidities, age, or drug regimens.
Thus, the door remains wide open for translational research and human trials.
Conclusion
The story of sildenafil continues to evolve. From a “failed antihypertensive” turned erectile miracle pill, it has now emerged as a subtle endocrine modulator of Leydig cell steroidogenesis. By orchestrating the cAMP and cGMP pathways, sildenafil enhances StAR protein activity and testosterone biosynthesis, offering new insights into the interconnectedness of vascular, reproductive, and endocrine physiology.
This discovery underscores a timeless lesson in medicine: drugs often act beyond their intended targets, and serendipity — when investigated rigorously — can expand therapeutic horizons.
For clinicians, it invites a broader perspective: sildenafil may not only restore erections but also strengthen the hormonal foundation upon which male vitality rests.
FAQ
1. Does sildenafil increase testosterone in men as well as in rats?
Some human studies report modest increases in serum testosterone during chronic PDE5 inhibitor use, but results are variable. More controlled trials are needed before drawing firm conclusions.
2. Can sildenafil replace testosterone therapy in hypogonadal men?
No. While it may enhance endogenous production, the effect is modest. Men with true hypogonadism typically require formal testosterone replacement. Sildenafil may serve as an adjunct, not a substitute.
3. Are there risks to increased testosterone from sildenafil use?
Potentially. While beneficial for energy, libido, and erectile function, elevated testosterone could exacerbate prostate growth or hematocrit levels in susceptible individuals. Clinical monitoring is essential.