Introduction
The intersection of diabetes mellitus and male reproductive dysfunction represents a troubling and often underrecognized clinical reality. For millions of men worldwide, diabetes does not merely compromise glucose metabolism—it also silently erodes sexual health and fertility. Erectile dysfunction, reduced libido, impaired spermatogenesis, and hormonal imbalances form a complex constellation of symptoms that can devastate quality of life and reproductive potential alike.
While conventional treatments—ranging from insulin therapy to phosphodiesterase inhibitors—address certain aspects of this dysfunction, they seldom restore the intricate physiological harmony of the hypothalamic-pituitary-gonadal (HPG) axis, which orchestrates male reproductive function.
Enter Lycium barbarum, better known as the goji berry. Revered for centuries in traditional Chinese medicine as a tonic for vitality, its bioactive component—Lycium barbarum polysaccharides (LBP)—has captured scientific interest for its antioxidative, neuroprotective, and anti-aging properties. Recent research now positions LBP as a potential restorative agent for diabetic-induced male sexual dysfunction, capable of reactivating the HPG axis and reviving testicular health.
This article explores the biological underpinnings of this phenomenon, examining how LBP acts at molecular and systemic levels to reverse diabetes-induced reproductive decline.
The Hidden Epidemic: Diabetes and Male Reproductive Dysfunction
The global rise of diabetes, particularly type 1 diabetes mellitus (T1DM), has turned a metabolic disorder into a multisystem crisis. Among its less discussed consequences is male infertility, a problem affecting nearly half of all diabetic men.
Hyperglycemia triggers a cascade of metabolic disturbances, including oxidative stress, mitochondrial dysfunction, and endocrine disruption. In the male reproductive system, this translates into:
- Testicular atrophy, due to oxidative damage and apoptosis of germ cells.
- Hormonal imbalance, with decreased testosterone and gonadotropin levels.
- Erectile and ejaculatory dysfunction, driven by endothelial damage and neuropathy.
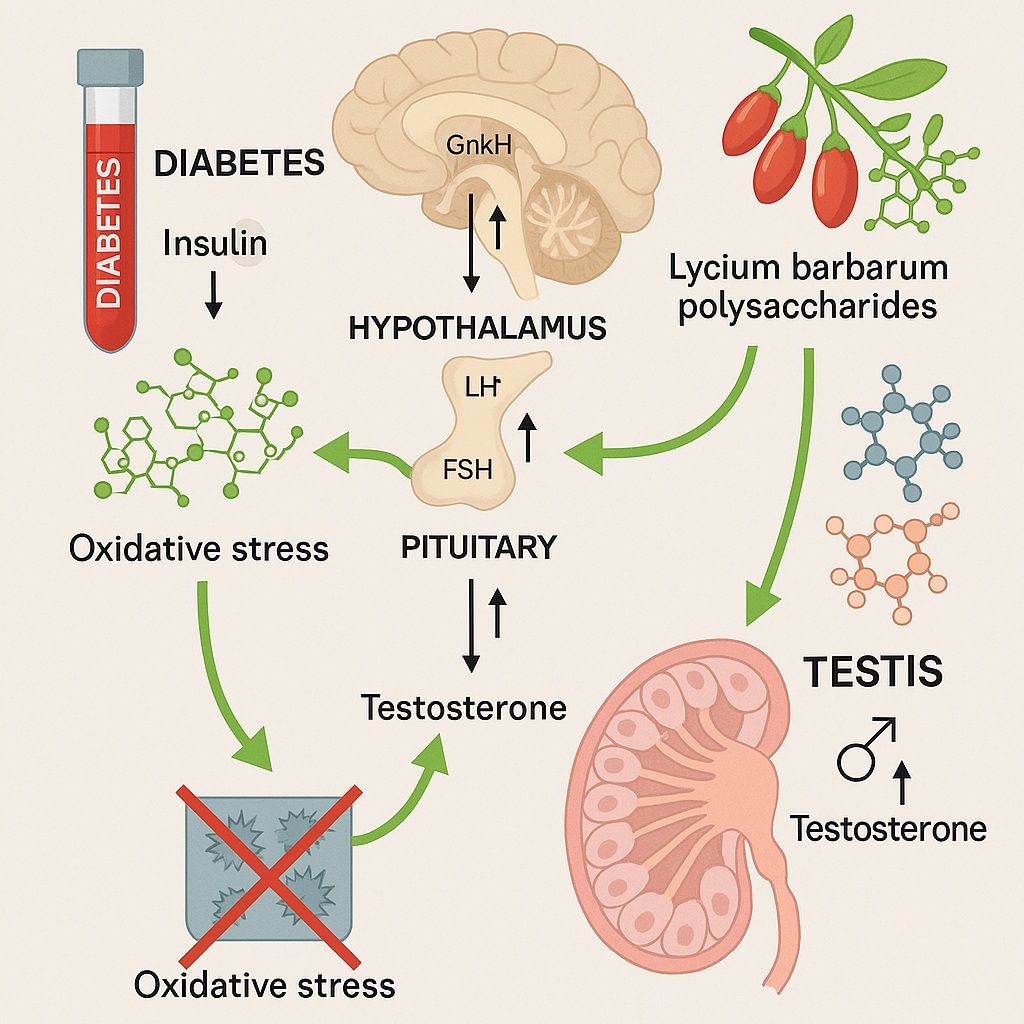
Compounding these physiological effects is the dysregulation of the HPG axis, the neuroendocrine network linking the hypothalamus, pituitary gland, and testes. Chronic hyperglycemia suppresses hypothalamic gonadotropin-releasing hormone (GnRH) secretion, reduces pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and ultimately curtails testosterone synthesis in Leydig cells.
Conventional diabetes management seldom addresses these reproductive consequences directly. Thus, restoring HPG axis activity remains a major unmet need in diabetic reproductive medicine—a gap that Lycium barbarum polysaccharides may help fill.
Meet Lycium barbarum: Nature’s Bioactive Powerhouse
Lycium barbarum, a perennial shrub native to China and parts of Southeast Asia, has earned the moniker “red diamond” in traditional medicine. Its bright orange-red berries are rich in polysaccharides, flavonoids, carotenoids, and amino acids, which collectively contribute to its therapeutic properties.
The polysaccharide fraction (LBP), representing approximately 5–8% of the dried fruit, is considered the primary bioactive component. Structurally, LBP comprises arabinose, glucose, galactose, mannose, and rhamnose, arranged in complex glycosidic linkages that interact with cellular receptors to trigger antioxidant, immunomodulatory, and endocrine effects.
Previous studies have shown that LBP:
- Enhances spermatogenesis and sperm motility.
- Protects against oxidative testicular injury induced by heat, radiation, or toxins.
- Improves sexual behavior and testosterone levels in animal models.
These diverse benefits make LBP a promising candidate for counteracting the multifactorial pathogenesis of diabetic reproductive dysfunction.
The Experimental Framework: Understanding the Mouse Model
To elucidate LBP’s role in male fertility restoration, researchers employed a streptozotocin (STZ)-induced T1DM mouse model. STZ, a pancreatic β-cell toxin, reliably induces hyperglycemia and mimics the endocrine and metabolic disturbances observed in human diabetes.
The experimental design divided male mice into four groups:
- Control group – healthy mice.
- Diabetic group – STZ-induced diabetic mice without treatment.
- LBP-treated diabetic group – diabetic mice administered LBP.
- Metformin-treated diabetic group – diabetic mice receiving standard antidiabetic therapy for comparison.
Over several weeks, the study assessed key parameters of reproductive health, including sexual behavior, testicular morphology, sperm count and motility, and hormonal levels (GnRH, LH, FSH, and testosterone). Molecular analyses further evaluated the expression of GnRH receptors, steroidogenic enzymes, and antioxidant proteins in the hypothalamus and testes.
The results revealed a striking pattern: LBP not only improved glucose tolerance but also rejuvenated the entire reproductive axis, surpassing the metabolic improvements achieved by metformin alone.
Reviving the HPG Axis: The Neuroendocrine Link
Central to LBP’s protective effect is its ability to reactivate the HPG axis, the master regulator of male reproductive physiology.
Hypothalamic Activation
In diabetic mice, hyperglycemia and oxidative stress suppress the hypothalamic release of GnRH, the hormone that initiates the cascade of gonadal regulation. LBP restored hypothalamic GnRH synthesis and secretion, as evidenced by elevated GnRH mRNA and protein levels.
This effect appears mediated through antioxidant and anti-inflammatory mechanisms. By scavenging reactive oxygen species (ROS) and inhibiting NF-κB signaling, LBP protects neuronal integrity in the hypothalamus, allowing normal neuroendocrine signaling to resume.
Pituitary Recovery
Once GnRH secretion is restored, downstream signaling follows. LBP-treated mice exhibited significant increases in LH and FSH levels, reflecting enhanced pituitary responsiveness. LH stimulates Leydig cells to produce testosterone, while FSH supports Sertoli cell function and spermatogenesis.
Testicular Restoration
At the terminal end of the axis, LBP normalized testosterone synthesis by upregulating steroidogenic acute regulatory protein (StAR) and cytochrome P450 side-chain cleavage enzyme (P450scc)—key mediators of steroidogenesis. These molecular adjustments translated into improved testicular histology, with restored seminiferous tubule architecture and higher sperm density.
Taken together, LBP effectively reboots the neuroendocrine circuitry disrupted by diabetes, achieving hormonal harmony that conventional glucose-lowering therapy fails to deliver.
Oxidative Stress and the Testicular Battlefield
The diabetic testis is a war zone of oxidative stress. Persistent hyperglycemia drives excessive ROS production, overwhelms antioxidant defenses, and damages lipids, proteins, and DNA within germ cells. This oxidative onslaught disrupts spermatogenesis, leading to decreased sperm viability and motility.
LBP acts as a molecular shield. Rich in hydroxyl groups, its polysaccharide structure allows it to directly scavenge free radicals. More importantly, LBP enhances endogenous antioxidant systems by:
- Increasing superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity.
- Reducing malondialdehyde (MDA), a lipid peroxidation marker.
- Inhibiting the NADPH oxidase complex responsible for ROS generation.
Histological analysis from the study showed that LBP-treated testes maintained intact seminiferous epithelium, regular germ cell arrangement, and robust spermatogenic layers, in stark contrast to the atrophic, vacuolated tubules observed in untreated diabetic mice.
This suggests that beyond endocrine restoration, LBP exerts a direct cytoprotective effect on testicular tissue integrity.
Reproductive Function Restored: Behavioral and Fertility Outcomes
Physiological recovery must translate into functional outcomes—and in this regard, LBP’s effects were equally impressive.
LBP-treated diabetic mice displayed significant improvements in sexual performance metrics, including:
- Shorter mounting and intromission latency.
- Increased copulatory frequency.
- Enhanced ejaculatory behavior.
Sperm analyses mirrored these behavioral gains, revealing higher sperm concentration, motility, and morphological normality. Moreover, LBP-treated males sired larger litter sizes when mated with healthy females, confirming functional restoration of fertility.
These behavioral results underscore a key point: LBP does not merely normalize biomarkers; it translates molecular recovery into reproductive competence—the true benchmark of therapeutic success in reproductive medicine.
Mitochondrial and Apoptotic Pathways: Inside the Cellular Machinery
Mitochondria are both the powerhouses and Achilles’ heels of testicular cells. Diabetes impairs mitochondrial function, leading to reduced ATP production and activation of apoptotic cascades.
LBP mitigates this by:
- Promoting mitochondrial biogenesis through PGC-1α upregulation.
- Maintaining mitochondrial membrane potential, preventing cytochrome c release.
- Modulating Bcl-2/Bax ratios, favoring cell survival.
Apoptosis-related protein analysis revealed that LBP suppressed caspase-3 activation, further confirming its anti-apoptotic role. By preserving mitochondrial integrity, LBP ensures that germ cells remain energy-efficient and resilient under diabetic stress.
Comparison to Conventional Therapies
Standard therapies for diabetic infertility—insulin regulation, metformin, and antioxidant supplementation—yield partial benefits at best. Metformin, while effective in lowering glucose, does not significantly improve testosterone synthesis or spermatogenic recovery.
In contrast, LBP uniquely targets both metabolic and reproductive pathways, integrating systemic glycemic control with endocrine and testicular protection. Its natural origin and low toxicity further enhance its appeal as a potential adjuvant or standalone therapy.
Although human clinical data remain limited, preclinical evidence positions LBP as a holistic regulator of male reproductive health, bridging traditional herbal wisdom with contemporary endocrinology.
Broader Implications: LBP Beyond Reproduction
Interestingly, LBP’s benefits extend well beyond the reproductive system. Its neuroprotective, immunomodulatory, and anti-aging properties have been validated across multiple models of oxidative and metabolic stress.
By modulating cellular signaling pathways such as PI3K/Akt, MAPK, and Nrf2, LBP enhances cellular resilience throughout the body. Its influence on mitochondrial dynamics, antioxidant defense, and hormonal regulation suggests a potential role in addressing other diabetes-associated complications—ranging from neuropathy to cognitive impairment.
Thus, LBP exemplifies a systems-level therapeutic: a single molecule capable of harmonizing interlinked physiological networks.
Conclusion
The research on Lycium barbarum polysaccharides presents an elegant convergence of traditional medicine and modern molecular biology. What ancient healers described as a vitality tonic now reveals itself, under the scrutiny of modern science, as a powerful modulator of endocrine and reproductive health.
By reactivating the HPG axis, reducing oxidative stress, and preserving testicular architecture, LBP effectively reverses diabetes-induced male reproductive dysfunction in animal models. Its integration of neuroendocrine, antioxidant, and mitochondrial mechanisms underscores a sophisticated pharmacological profile that conventional therapies lack.
While translation to human clinical use requires further validation, the evidence so far is compelling. In the quest to restore fertility and vitality in diabetic men, Lycium barbarum may not just supplement treatment—it may redefine it.
FAQ: Lycium barbarum Polysaccharides and Male Reproductive Health
1. How does diabetes affect male fertility?
Diabetes disrupts the hypothalamic-pituitary-gonadal axis, reduces testosterone levels, damages sperm via oxidative stress, and impairs sexual performance through vascular and neural dysfunction.
2. What makes Lycium barbarum polysaccharides effective in this context?
LBP acts on multiple fronts—it reduces oxidative stress, restores GnRH–LH–FSH signaling, enhances testosterone synthesis, and protects testicular cells from apoptosis, collectively revitalizing reproductive function.
3. Can LBP be used clinically to treat human infertility?
Human trials are limited, but preclinical data are promising. With further validation, standardized LBP extracts could emerge as a safe, natural adjunct in managing diabetes-related male infertility.