Introduction: A Paradigm Shift in Oncology
In modern oncology, the pursuit of effective therapies is often constrained by both time and cost. Developing a single novel anticancer drug can take over a decade and exceed two billion dollars in investment — with a daunting failure rate during clinical translation. Amid these realities, an elegant solution has emerged: drug repurposing, or the strategic redeployment of existing non-oncology agents for cancer therapy.
Repurposing leverages what medicine already knows — the pharmacodynamics, pharmacokinetics, and safety profiles of approved drugs — to confront the molecular complexity of cancer. Unlike conventional drug discovery, which starts with an uncharted compound, repurposing begins with a molecule that has already proven itself biocompatible in humans. This approach not only reduces development costs and timeframes but also facilitates rapid clinical translation, particularly when preclinical data support new therapeutic indications.
In the past decade, repurposing has transformed from a sporadic act of clinical curiosity into a methodologically rigorous field, powered by advances in bioinformatics, systems biology, and precision pharmacology. Drugs once confined to cardiology, endocrinology, or psychiatry are now being evaluated for their capacity to modulate oncogenic signaling, reverse immune suppression, and improve tumor microenvironmental resilience.
This article explores how this strategy is reshaping cancer therapy — its current landscape, pharmacological rationale, and the future directions that could make repurposing one of the most impactful revolutions in oncology.
The Scientific Rationale: Molecular Overlaps Between Chronic Disease and Cancer
At its core, cancer is not a singular disease but a systemic dysregulation of cellular signaling, metabolism, and survival. This complexity creates molecular intersections between cancer and other chronic conditions — cardiovascular disease, diabetes, neurodegeneration, and inflammation — where existing drugs already act.
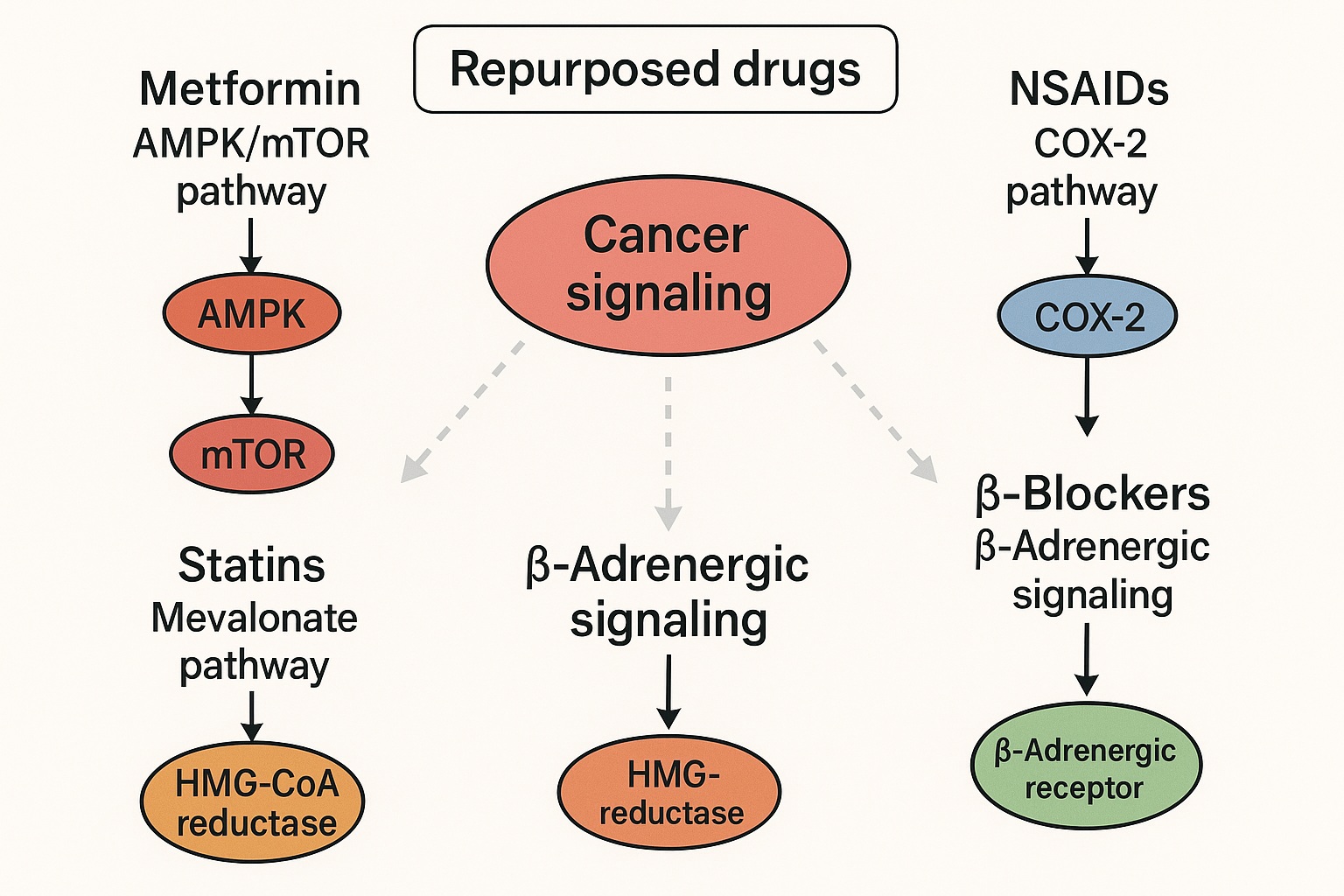
Many non-oncology drugs exert pleiotropic effects that incidentally target oncogenic processes. Statins, designed to control cholesterol biosynthesis, inhibit HMG-CoA reductase, a key enzyme also involved in the synthesis of isoprenoid intermediates critical for post-translational modification of RAS proteins — one of the most prominent oncogenic families in human cancers.
Similarly, metformin, a frontline antidiabetic agent, activates the AMP-activated protein kinase (AMPK) pathway and inhibits mammalian target of rapamycin (mTOR) signaling — both central to tumor metabolism and growth. Beta-blockers modulate adrenergic signaling, curbing stress-induced angiogenesis and metastatic potential. Even nonsteroidal anti-inflammatory drugs (NSAIDs), long used for pain and arthritis, suppress the COX-2/PGE2 inflammatory cascade, which plays a pivotal role in tumor initiation, proliferation, and immune evasion.
The overlap is not coincidental. These mechanistic bridges between chronic disease pathways and oncogenesis represent fertile ground for pharmacologic innovation. In essence, repurposing exploits the shared molecular grammar of disease — a concept that continues to redefine therapeutic boundaries.
Pharmacologic Categories with Oncologic Promise
While dozens of non-oncology agents have shown anticancer potential, several pharmacologic classes stand out for their mechanistic depth and clinical evidence.
1. Metabolic Regulators
Metformin remains the poster child of drug repurposing in oncology. Beyond glycemic control, it exerts profound metabolic stress on cancer cells by decreasing ATP production via mitochondrial complex I inhibition. This leads to activation of AMPK, inhibition of mTORC1, and suppression of protein and lipid synthesis. Moreover, metformin reduces systemic insulin and insulin-like growth factor (IGF-1) levels, depriving tumors of a key proliferative signal.
Preclinical and epidemiologic studies show that metformin may lower cancer incidence and improve survival in diabetic patients, particularly in breast, prostate, and colorectal cancers. Current trials are examining its adjuvant use with chemotherapy and immunotherapy, exploiting its potential to reprogram cancer metabolism and enhance treatment sensitivity.
2. Cardiovascular Agents
Statins and beta-blockers exemplify how drugs born in cardiology can find new purpose in oncology. Statins’ ability to impair mevalonate pathway flux disrupts the prenylation of small GTPases such as RAS, RAC, and RHO — effectively blunting oncogenic signaling and inhibiting metastasis.
Beta-blockers, particularly non-selective ones like propranolol, exert antitumor activity through modulation of β-adrenergic receptor signaling. Chronic adrenergic stimulation promotes angiogenesis, immune suppression, and epithelial-mesenchymal transition (EMT); blocking this axis can therefore diminish tumor vascularity and metastatic spread. Clinical data suggest improved outcomes in patients with breast, ovarian, and melanoma when treated concomitantly with beta-blockers.
3. Anti-inflammatory and Immunomodulatory Drugs
The link between inflammation and cancer is well established, and NSAIDs have long attracted attention for their chemopreventive properties. By inhibiting COX-2, NSAIDs reduce prostaglandin-mediated angiogenesis, immune modulation, and tumor invasiveness. Regular aspirin use, for instance, has been associated with reduced incidence of colorectal and esophageal cancers.
Glucocorticoids, though immunosuppressive, have paradoxical roles: in hematologic malignancies, they induce apoptosis via glucocorticoid receptor activation. Meanwhile, anti-rheumatic agents such as chloroquine and hydroxychloroquine are being explored for their ability to inhibit autophagy, a cellular process cancer cells exploit for survival under stress.
4. Neuropsychiatric Drugs
Some antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs) and tricyclics, exhibit cytotoxicity in certain tumor lines. Tricyclics like clomipramine inhibit mitochondrial complex III, leading to ROS accumulation and apoptotic cell death. Additionally, antipsychotics such as thioridazine can induce differentiation in cancer stem-like cells, disrupting tumor maintenance.
While enthusiasm here remains largely preclinical, these findings illustrate the surprising diversity of pharmacologic strategies hidden within the existing pharmacopeia.
The Systems Biology Approach: Mapping Repurposing Opportunities
Repurposing today is no longer an act of serendipity — it’s an algorithm-driven science. The convergence of omics technologies (genomics, proteomics, metabolomics) and network pharmacology enables systematic identification of drug–disease intersections.
Computational pipelines now mine databases like DrugBank, LINCS, and Connectivity Map (CMap) to match drug-induced transcriptional signatures with cancer-specific gene expression profiles. If a non-oncology drug produces a gene expression pattern that inversely correlates with that of a tumor, it becomes a candidate for repurposing.
These in silico tools are complemented by artificial intelligence (AI) and machine learning (ML) platforms that predict off-target binding, polypharmacology, and synergy with standard therapies. The result is a high-throughput discovery model that compresses years of laboratory screening into months of computational analysis.
Importantly, this approach reflects a conceptual shift: instead of pursuing single targets, researchers are embracing network pharmacology, recognizing that complex diseases like cancer demand multi-nodal modulation. Drugs with pleiotropic effects — once dismissed for their “dirty” pharmacology — are now valued for their ability to correct multiple aberrant pathways simultaneously.
Clinical Evidence: Translating Repurposing into Practice
Clinical validation remains the defining hurdle for repurposed drugs. While preclinical studies abound, relatively few have matured into phase III clinical trials. However, the past decade has seen notable progress.
Metformin has entered dozens of trials as an adjunct to chemotherapy, radiotherapy, and immunotherapy. Results vary, but a meta-analysis of diabetic breast cancer patients suggests improved overall and progression-free survival.
Propranolol has demonstrated activity against angiosarcoma, where β-adrenergic signaling drives vascular proliferation. In combination with chemotherapy, propranolol enhanced tumor regression and prolonged remission, representing one of the few successful real-world examples of oncologic repurposing.
Aspirin continues to be evaluated for secondary prevention in colorectal and pancreatic cancers, with growing support for its integration into chemoprevention protocols for high-risk populations.
Nevertheless, the translation pipeline is slow. Repurposed drugs, being off-patent, lack the commercial incentives that drive large-scale phase III trials. Consequently, many promising agents linger in the limbo of early-phase research — scientifically compelling but economically orphaned.
Challenges and Limitations: The Double-Edged Sword of Accessibility
Despite its promise, drug repurposing faces substantial scientific, regulatory, and financial barriers.
Scientific Heterogeneity
Mechanisms observed in vitro do not always translate to human efficacy. Dose adjustments are often necessary, as anticancer effects may require concentrations exceeding those safely achieved for the original indication. Furthermore, many repurposed drugs act indirectly — modulating the tumor microenvironment rather than targeting cancer cells themselves — complicating biomarker development and efficacy assessment.
Regulatory Uncertainty
The regulatory framework for repurposing remains fragmented. Agencies such as the FDA and EMA allow label expansion for new indications, but the process requires robust efficacy data equivalent to that for new drugs. For academic institutions and small biotech firms, such trials are prohibitively costly.
Economic Disincentives
The absence of intellectual property protection for off-patent drugs creates a paradox: the more accessible a drug is, the less incentive there is for industry to invest in its new clinical development. This has given rise to “financial orphan drugs” — molecules with enormous therapeutic potential but little commercial viability.
To overcome these barriers, researchers have called for public–private partnerships, nonprofit consortia, and government-backed incentives to support repurposing initiatives. The success of such collaborations in infectious diseases (e.g., tuberculosis and COVID-19) provides a model worth emulating in oncology.
Combination Therapies: Synergy Between Old and New
One of the most promising applications of repurposing lies in combination therapy. Cancer’s hallmark heterogeneity demands multitargeted strategies, and repurposed drugs often exhibit additive or synergistic effects with standard treatments.
- Metformin + Chemotherapy: enhances apoptosis by suppressing mitochondrial energy metabolism and reducing tumor hypoxia.
- Statins + Immunotherapy: modulate tumor immune evasion by altering membrane cholesterol composition and enhancing T-cell infiltration.
- NSAIDs + Checkpoint Inhibitors: reduce inflammation-driven immunosuppression, potentially improving the efficacy of PD-1/PD-L1 blockade.
These synergies extend beyond pharmacodynamics. Repurposed agents can mitigate the toxicities of standard regimens — for example, beta-blockers reducing chemotherapy-induced cardiotoxicity, or metformin protecting against metabolic dysregulation from hormone therapies.
Ethical and Global Implications
Repurposing also represents a form of therapeutic democratization. Many repurposed drugs are inexpensive and widely available, making them particularly attractive for low- and middle-income countries, where access to cutting-edge oncology therapeutics remains limited.
However, ethical considerations persist. Using a repurposed drug outside its approved indication demands careful informed consent, transparent discussion of risks, and ideally, inclusion within structured clinical research frameworks. Compassionate use must not become a substitute for evidence generation.
Moreover, as personalized oncology advances, the ethical obligation grows to identify which patient subsets truly benefit. Repurposing cannot afford to replicate the one-size-fits-all failures of past oncology paradigms.
The Future of Drug Repurposing: From Opportunism to Precision
The next frontier of repurposing lies in precision medicine — integrating molecular profiling, pharmacogenomics, and AI-driven modeling to tailor repurposed therapies to individual tumor biology.
Multi-omics datasets will help identify predictive biomarkers that stratify patients likely to respond to specific non-oncology drugs. Meanwhile, advances in organoid modeling and patient-derived xenografts (PDXs) will enable preclinical validation in physiologically relevant systems.
On the policy front, regulatory bodies are beginning to recognize the unique potential of repurposing. Initiatives like the FDA’s “New Uses of Old Drugs” program and the EMA’s Adaptive Pathways framework are early steps toward creating a formal infrastructure for accelerated approval.
Finally, the integration of machine learning into clinical trial design will allow adaptive, real-time modification of dosing and endpoints — a paradigm that may cut years off the conventional trial timeline.
Conclusion: The Quiet Revolution of Rational Repurposing
Drug repurposing is not a romantic notion of medical serendipity; it is an evidence-based, strategically essential approach to modern cancer therapy. By harnessing the pharmacologic versatility of existing molecules, it bridges the gap between unmet clinical needs and sustainable innovation.
While challenges remain — particularly in regulatory alignment and economic motivation — the cumulative evidence is compelling. The same molecules that once tamed cholesterol, hypertension, or diabetes may soon redefine the therapeutic architecture of oncology.
The task ahead is not merely to repurpose drugs, but to repurpose the scientific and institutional mindset that governs how we value innovation. The future of oncology may very well depend on how efficiently we learn to look backward — to move forward.
FAQ
1. Why are non-oncology drugs being considered for cancer therapy?
Because many chronic disease drugs target molecular pathways — such as metabolism, inflammation, and angiogenesis — that overlap with cancer biology. This shared molecular architecture allows existing drugs to exert anticancer effects through secondary mechanisms.
2. Which repurposed drugs show the strongest evidence of benefit in cancer?
Metformin, statins, beta-blockers, and NSAIDs currently have the most robust preclinical and clinical data, particularly in breast, colorectal, and prostate cancers. However, their effects vary by tumor type and patient population.
3. What are the biggest challenges in translating drug repurposing to clinical practice?
Key challenges include limited commercial incentives for off-patent drugs, the need for high-quality phase III trials, and the regulatory complexity of approving new indications for existing agents. Overcoming these barriers requires collaborative funding models and supportive policy frameworks.