Introduction
For over two decades, sildenafil citrate has been the pharmacological cornerstone of erectile dysfunction (ED) therapy. Yet, despite its clinical success, its traditional oral tablet formulation has remained imperfect. Patients and clinicians alike have long wrestled with three fundamental limitations: delayed onset of action, low bioavailability, and gastrointestinal side effects. The root causes are largely physicochemical — poor aqueous solubility and significant first-pass hepatic metabolism. These attributes make sildenafil a drug that works well in theory but occasionally underperforms in practice.
The search for an optimized delivery system that could bypass these limitations led researchers to a creative marriage between solid dispersion technology and fast orodissolvable film (ODF) formulations. In a study by Hosny et al. (2016), this concept materialized into an elegant solution: a thin polymeric film that dissolves almost instantly on the tongue, delivering sildenafil directly into the systemic circulation through the oral mucosa. The result — an 8-fold improvement in solubility and a 2.25-fold increase in bioavailability compared to standard tablets — was more than a pharmacokinetic curiosity; it represented a meaningful therapeutic advance.
This article explores the science, formulation design, and clinical implications of this optimized sildenafil ODF, a formulation that transforms a time-sensitive therapy into a fast, patient-friendly innovation.
The Persistent Barriers of Conventional Sildenafil Therapy
The standard sildenafil tablet, while effective, is inherently constrained by its physicochemical nature. The compound is practically insoluble in water and undergoes extensive hepatic metabolism via cytochrome P450 3A4, reducing its oral bioavailability to around 40%. Its absorption is also significantly affected by food, particularly high-fat meals that delay gastric emptying and slow onset of effect.
From a pharmacokinetic perspective, the typical onset of action (Tmax) occurs roughly 60 minutes post-ingestion, which is clinically inconvenient for a condition defined by spontaneity. Moreover, the tablet’s systemic absorption pathway predisposes patients to dyspepsia, flushing, and gastric discomfort, side effects that contribute to poor compliance and early treatment discontinuation.
To address these limitations, modern formulation science has looked beyond chemistry and into delivery design. The goal: increase the rate and extent of sildenafil absorption while bypassing gastrointestinal and hepatic bottlenecks — ideally, without altering the molecule itself.
The Rationale Behind Orodispersible Film Technology
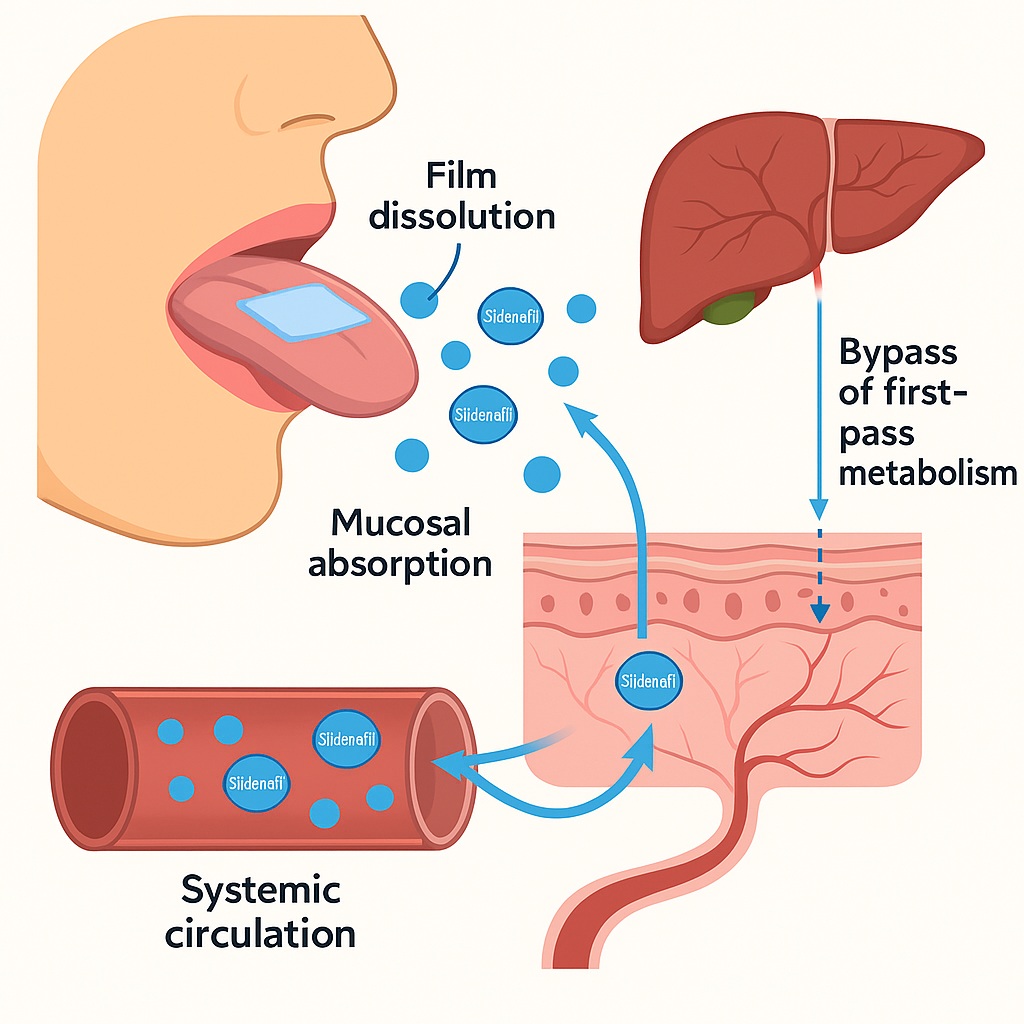
The fast orodissolvable film (ODF) represents one of the most patient-centric dosage forms developed in recent years. Thin, flexible, and approximately the size of a postage stamp, these films disintegrate within seconds when placed on the tongue, releasing the active drug for rapid mucosal absorption. The formulation circumvents first-pass metabolism, potentially leading to faster onset and higher systemic availability.
Beyond pharmacokinetics, ODFs provide distinct clinical and behavioral advantages:
- Ease of administration without water — ideal for patients with dysphagia or mobility limitations.
- Enhanced compliance and discretion, particularly relevant for ED patients reluctant to take tablets in social settings.
- Reduced gastrointestinal irritation, since the film’s absorption occurs primarily through the oral mucosa.
However, crafting a viable ODF is far from trivial. The film must balance mechanical strength, rapid disintegration, drug uniformity, and pleasant mouthfeel — all while accommodating the physicochemical quirks of the active ingredient. Sildenafil citrate, with its low solubility and bitterness, presents a formidable challenge.
Cyclodextrin Complexation: A Solubility Breakthrough
At the molecular level, the research team tackled sildenafil’s solubility problem using cyclodextrin-based inclusion complexes. Cyclodextrins (CDs) are cyclic oligosaccharides with a hydrophobic central cavity and hydrophilic outer surface — a molecular architecture that enables them to “host” lipophilic drug molecules inside their cavity, thereby improving apparent solubility and stability.
Three derivatives were evaluated:
- β-cyclodextrin,
- hydroxypropyl-β-cyclodextrin, and
- hydroxybutyl-β-cyclodextrin (HBβCD).
Phase-solubility analysis revealed a 1:1 stoichiometric complex between sildenafil and each cyclodextrin type, but hydroxybutyl-β-cyclodextrin emerged as the superior candidate, enhancing solubility by up to ninefold. Its stability constant (954 M⁻¹) was substantially higher than the other derivatives, likely due to the hydrophobic alkyl chains that strengthened inclusion interactions.
When incorporated via solid dispersion using the coevaporation method, the sildenafil–HBβCD complex achieved aqueous solubility of 24.33 mg/mL, compared to a mere 3.38 mg/mL for pure drug. This transformation laid the foundation for a film capable of immediate disintegration and rapid systemic uptake.
The Box–Behnken Design: Statistical Elegance in Formulation Optimization
Rather than relying on trial-and-error formulation, the investigators employed the Box–Behnken experimental design, a statistical model that allows systematic optimization of multiple variables with minimal experimental runs. Three key factors were selected:
- X₁: Hydroxypropyl cellulose (HPC) — film-forming polymer
- X₂: Guar gum — film modifier
- X₃: Propylene glycol — plasticizer
The design evaluated their effects on two critical responses:
- Y₁: Disintegration time (to be minimized)
- Y₂: Drug release percentage (to be maximized)
Fifteen formulations were prepared and analyzed for mechanical and dissolution characteristics. The statistical model revealed clear relationships among the variables: increasing polymer concentration prolonged disintegration and reduced drug release, while increasing plasticizer content shortened disintegration time and enhanced release. The modifier (guar gum) showed minimal effect, confirming its role as a structural stabilizer rather than a performance driver.
This approach yielded an optimized formula (HPC 1%, guar gum 0.5%, propylene glycol 2%) with a disintegration time of 89 seconds and 86% drug release within minutes — a balance of strength, flexibility, and functional efficiency rarely achieved in orodispersible films.
Fabrication of the Orodispersible Film: From Laboratory to Lingual Delivery
The optimized film was prepared via the solvent casting method. The polymeric base (HPC and guar gum) was hydrated in distilled water and allowed to stand for 24 hours to remove air bubbles. The drug–cyclodextrin solid dispersion, plasticizer, and a small quantity of aspartame (for taste masking) were dissolved in ethanol-water solution and incorporated into the polymer matrix.
The resulting viscous solution was poured into glass Petri dishes, dried, and cut into uniform 2 × 2.5 cm strips, each containing 50 mg sildenafil citrate. These translucent, flexible strips were mechanically robust, with folding endurance exceeding 300 cycles — a critical parameter ensuring that films do not crack during handling or packaging.
Physicochemical evaluation confirmed uniform thickness, consistent drug content, and excellent surface morphology, validating the precision of the optimized process.
In Vitro Performance: Speed, Release, and Structural Integrity
Disintegration testing demonstrated that the films began to dissolve within 1.5 minutes, a vast improvement over tablet dissolution times exceeding 15 minutes. The in vitro dissolution studies further supported these findings, showing 86% of sildenafil released within 30 minutes, a release profile consistent with rapid absorption through the oral mucosa.
Statistical modeling indicated that polymer concentration exerted the most significant control over disintegration, whereas plasticizer concentration was the dominant factor influencing drug release kinetics. These insights underline the delicate balance between film rigidity and wettability, both of which determine clinical performance.
Pharmacokinetic Brilliance: Rapid Absorption and Enhanced Bioavailability
The ultimate test of formulation innovation lies in its in vivo performance. A double-blind, randomized, crossover study involving twelve healthy male volunteers compared the pharmacokinetics of the optimized ODF (50 mg sildenafil) to that of the marketed Viagra® tablet.
The results were striking:
| Parameter | Viagra Tablet | Optimized ODF |
|---|---|---|
| Cmax (ng/mL) | 215 ± 20 | 545 ± 31 |
| Tmax (hours) | 1.25 ± 0.1 | 0.4 ± 0.07 |
| AUC₀₋₂₄ (ng·h/mL) | 1135 ± 165 | 2205 ± 206 |
| Relative Bioavailability | 100% | 225% |
In practical terms, the ODF achieved therapeutic plasma concentrations within 10 minutes, compared to 60 minutes for the standard tablet. This 6-fold acceleration in onset transforms patient experience, enabling near-spontaneous sexual readiness. Moreover, the 2.25-fold increase in bioavailability suggests that lower doses could achieve equivalent efficacy, potentially reducing side effects and cost per treatment.
Mechanistic Insights: Why the Film Outperforms the Tablet
The enhanced pharmacokinetic profile of the ODF stems from multiple intertwined mechanisms:
- Bypassing First-Pass Metabolism: Absorption through the rich vascular network of the oral mucosa delivers sildenafil directly to systemic circulation, avoiding hepatic degradation.
- Improved Solubility and Dissolution: The hydroxybutyl-β-cyclodextrin complex keeps sildenafil in a readily soluble, amorphous state, eliminating dissolution lag.
- Reduced Gastric Variability: Since absorption occurs pre-gastrically, factors like pH, gastric motility, and food intake no longer interfere.
- Rapid Onset via Buccal Membrane Diffusion: The thin mucosal barrier and high perfusion rate ensure quick systemic delivery.
Collectively, these mechanisms elevate the ODF beyond a simple convenience product; it becomes a pharmacokinetic optimization platform, capable of reshaping the therapeutic window of lipophilic oral drugs.
Patient-Centered Advantages: Beyond Pharmacology
From a clinical perspective, the benefits of sildenafil ODF extend far beyond absorption metrics. Patients often report hesitation in taking tablets before intimacy, both for psychological and logistical reasons. The film’s discreet and portable nature minimizes social embarrassment and enhances spontaneity — intangible but powerful determinants of adherence.
Furthermore, ODFs are ideal for populations with swallowing difficulties, such as elderly men or those with post-stroke dysphagia. The pleasant mouthfeel and absence of water requirement further improve user satisfaction. For healthcare providers, this translates into improved compliance, fewer missed doses, and more predictable therapeutic outcomes.
Challenges and Future Directions
Despite its promise, ODF technology for sildenafil is not without challenges. Manufacturing requires precise environmental control to maintain film uniformity and prevent moisture-induced degradation. Sildenafil’s intrinsic bitterness also demands effective taste masking strategies, such as sweeteners or flavoring agents, without compromising mucosal absorption.
Moreover, large-scale production introduces regulatory complexities regarding dose uniformity, mechanical integrity, and stability under variable humidity. These hurdles, while non-trivial, are surmountable through continued research in polymer science, nanocarrier systems, and packaging technology.
Looking ahead, the principles established in this study could extend to other lipophilic, first-pass–limited drugs — from antihypertensives to psychotropics — marking a broader shift toward mucosal delivery as a mainstream pharmaceutical strategy.
The Broader Implication: Pharmaceutical Innovation through Design
The optimized sildenafil ODF exemplifies how formulation science can rescue a mature molecule from its limitations. The innovation did not alter sildenafil’s chemistry, yet it fundamentally redefined its therapeutic performance. This underscores a vital truth in modern pharmaceutics: that the future of drug development lies not only in discovering new molecules but in re-engineering how existing ones are delivered.
The use of statistical design tools like Box–Behnken methodology represents the new scientific rigor in formulation — replacing empirical tinkering with predictive modeling. By quantifying the relationships among excipients, film parameters, and performance outcomes, scientists can design dosage forms that are both rational and reproducible.
Such integration of biopharmaceutics, material science, and pharmacokinetics heralds a new era where dosage form innovation directly enhances therapeutic value.
Conclusion
The development of an optimized sildenafil citrate fast orodissolvable film represents a compelling advance in the management of erectile dysfunction. Through intelligent formulation — merging cyclodextrin-based solubilization, solid dispersion technology, and response surface optimization — researchers achieved a product with:
- Eightfold improved solubility,
- Rapid disintegration within 90 seconds,
- Therapeutic plasma levels achieved in under 10 minutes, and
- Bioavailability increased by 225% compared to conventional tablets.
Clinically, this translates into faster action, fewer gastrointestinal side effects, and greater patient confidence — all achieved without altering the drug’s fundamental pharmacodynamics.
In an era where pharmaceutical innovation often means chasing new molecules, the sildenafil ODF reminds us that the smartest advances may come from rethinking the old ones. Sometimes, true progress lies not in reinventing chemistry but in perfecting delivery.
FAQ: Sildenafil Orodispersible Films
1. How is the sildenafil orodispersible film different from regular tablets?
The ODF dissolves rapidly on the tongue and allows the drug to be absorbed through the oral mucosa, bypassing the liver’s first-pass metabolism. This results in faster onset, higher bioavailability, and reduced gastrointestinal side effects.
2. Does the film act faster than Viagra?
Yes. Plasma concentrations equivalent to tablet efficacy are achieved within about 10 minutes — compared to approximately 60 minutes with standard Viagra tablets.
3. Is this formulation suitable for all patients with erectile dysfunction?
Clinically, it offers advantages for patients seeking rapid onset and those with difficulty swallowing tablets. However, as with all sildenafil products, it must be used under medical supervision, particularly in individuals with cardiovascular conditions.