Introduction
The pharmaceutical industry has long prided itself on precision — in chemistry, formulation, and quality assurance. Yet, beneath the veneer of technological maturity lies an inconvenient truth: traditional tablet manufacturing remains largely 2D, constrained by compression, coating, and conventional layering techniques developed over half a century ago.
As the demand for personalized medicine and on-demand drug manufacturing accelerates, these limitations have become increasingly visible. In response, three-dimensional (3D) printing, or additive manufacturing, has emerged as a disruptive technology with the potential to revolutionize solid dosage form production.
The study “3D printed furosemide and sildenafil tablets: Innovative production and quality control” represents one of the most sophisticated explorations of this technology in pharmaceutical manufacturing. By combining two pharmacologically distinct molecules — furosemide, a loop diuretic, and sildenafil citrate, a PDE5 inhibitor — the researchers not only demonstrated the technical feasibility of multi-drug 3D printing but also tackled the crucial challenge of quality control in a highly customized, non-linear manufacturing process.
This article unpacks the essence of that innovation: the scientific rationale, the technological advances, and the regulatory implications of bringing 3D printing from experimental promise to industrial practice.
The Paradigm Shift: From Compression to Layer-by-Layer Construction
Traditional tablet production depends on granulation, drying, blending, and compression, followed by coating and packaging. These stages are sequential, rigid, and highly optimized for mass production, not for customization. In contrast, 3D printing constructs tablets layer by layer, using digital blueprints that allow for unprecedented control over the tablet’s geometry, porosity, and internal microarchitecture.
This process allows precise manipulation of drug loading, release kinetics, and mechanical strength. By altering the printing pattern or polymer composition, manufacturers can fine-tune disintegration time, diffusion rate, and surface-to-volume ratio — all within a single digital file.
The result is a new pharmaceutical reality: the ability to manufacture small batches or even single-unit formulations tailored to individual patient needs. For diseases requiring dynamic dosing (heart failure, pulmonary hypertension) or for drugs with narrow therapeutic windows, this capacity represents a radical departure from traditional bulk manufacturing.
In the study, furosemide and sildenafil served as model compounds precisely because their physicochemical and therapeutic characteristics are contrasting. Furosemide’s poor solubility and short half-life contrast with sildenafil’s lipophilicity and sensitivity to moisture. These differences made them ideal candidates for testing the adaptability of 3D printing technologies to multiple pharmacological profiles.
The Printing Process: Engineering Complexity at a Micron Scale
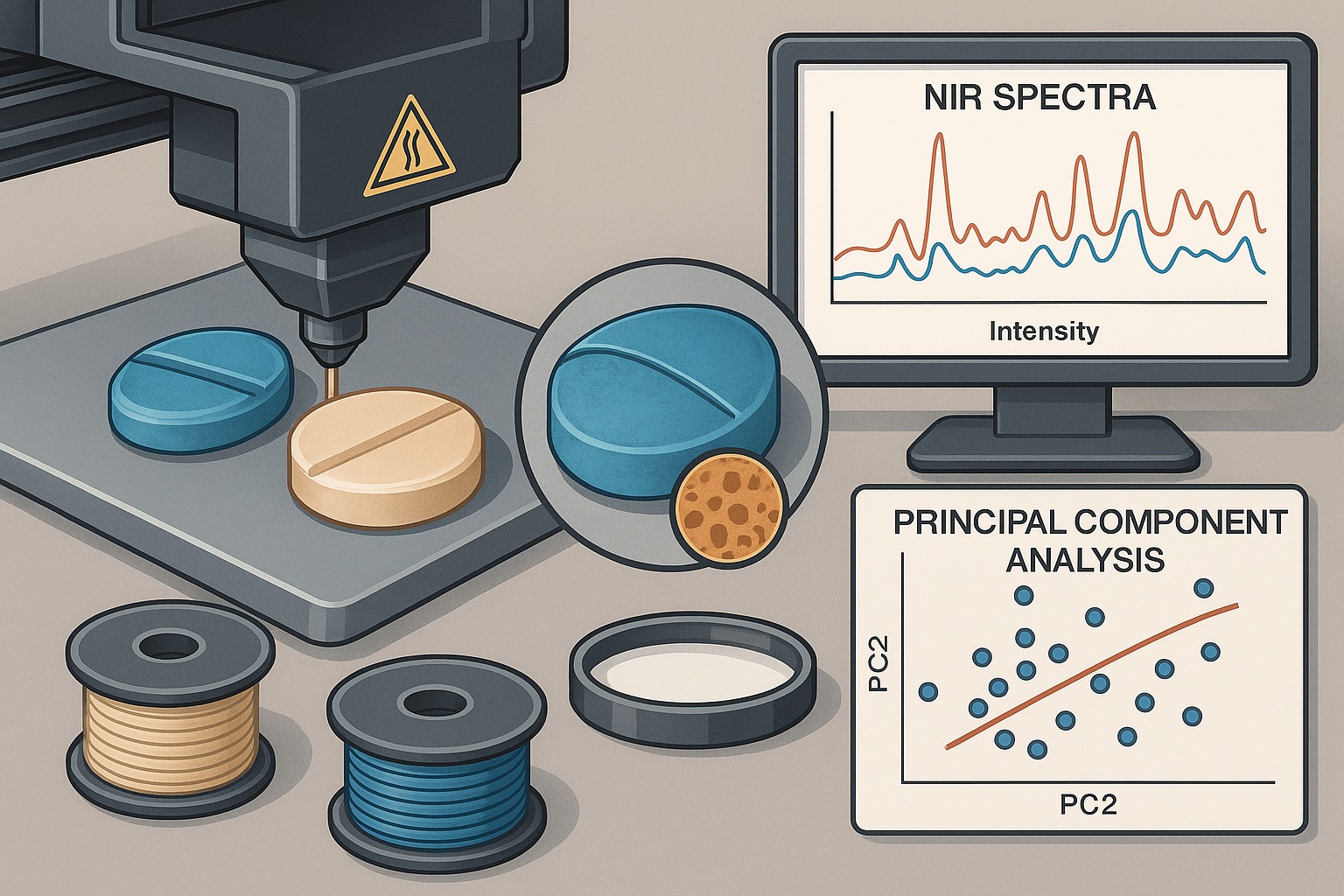
The research employed fused deposition modeling (FDM) — a 3D printing technique that extrudes thermoplastic polymer filaments through a heated nozzle, depositing material layer by layer to build the desired tablet geometry.
The process required first fabricating filaments loaded with the active pharmaceutical ingredients (APIs) via hot-melt extrusion (HME), followed by controlled deposition under computer-assisted design parameters. Each step demanded careful optimization of:
- Polymer matrix selection (e.g., polyvinyl alcohol and polyethylene glycol blends) for drug compatibility and printability.
- Thermal parameters, ensuring the temperature was high enough for polymer flow but below the degradation threshold of the APIs.
- Extrusion rate and nozzle diameter, directly influencing mechanical strength and drug distribution.
Notably, the team demonstrated that API stability was preserved throughout the printing process — a pivotal finding given the known thermal sensitivity of sildenafil. This was verified via Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC), which confirmed the absence of degradation or polymorphic transitions post-printing.
Moreover, the digital nature of the process allowed precise control over the tablet’s internal architecture. The researchers designed honeycomb-like porous structures to optimize drug release, creating a uniform matrix that balanced dissolution rate with mechanical integrity — a feat virtually impossible to replicate through compression alone.
Integrating Dual Drugs: A Masterclass in Pharmaceutical Design
Printing furosemide and sildenafil in one tablet was not merely an engineering exercise; it was a test of whether multi-drug printing could achieve controlled release without compromising either compound’s pharmacokinetic profile.
Two key approaches were explored:
- Co-extrusion — incorporating both APIs within a single polymer filament.
- Sequential printing — printing separate layers or compartments for each drug, allowing spatial and temporal control of release.
The second approach proved superior. By spatially isolating the APIs within different regions of the tablet, drug–drug interactions were minimized, and release kinetics could be modulated independently. This strategy mimics fixed-dose combination therapy, but with greater flexibility: the ratio, release order, and compartmental geometry can be digitally adjusted within minutes.
Mechanistically, furosemide was printed in a more hydrophilic matrix to promote rapid release, while sildenafil was embedded in a hydrophobic domain to prolong dissolution — effectively producing a dual-phase therapeutic tablet. Such intricate design would be nearly impossible using conventional compression technology.
The concept heralds a future where polypills — combinations of multiple drugs for cardiovascular or metabolic disorders — can be digitally personalized based on patient pharmacogenetics, organ function, and adherence profiles.
Quality Control Reinvented: Analytical Precision for Printed Pharmaceuticals
No innovation in pharmaceutical manufacturing can advance without robust quality control (QC). The study recognized this early and addressed a key regulatory bottleneck: how to ensure uniformity, reproducibility, and regulatory compliance when every printed tablet might be unique.
The researchers implemented an integrated QC workflow combining imaging, spectroscopy, and chemometric modeling:
- Near-infrared (NIR) spectroscopy was used to non-destructively assess drug distribution and homogeneity within tablets.
- Principal component analysis (PCA) and partial least squares (PLS) regression correlated spectral signatures with API concentration and physical properties.
- Micro-computed tomography (micro-CT) provided detailed visualization of internal porosity and structural uniformity.
Together, these tools formed a multi-dimensional QC paradigm, capable of detecting printing anomalies, filament inconsistencies, or suboptimal mixing at a micron level.
This approach shifts QC from batch-based sampling to real-time, data-driven assurance. The convergence of 3D printing with spectroscopic analytics embodies the Industry 4.0 vision of continuous manufacturing, where each unit is individually verifiable rather than statistically inferred from a batch.
Stability and Performance: When Precision Meets Reliability
Drug stability remains a critical determinant of clinical safety and shelf-life. One of the greatest concerns in additive manufacturing is the thermal degradation of APIs and polymers during extrusion and printing.
Extensive testing showed that both furosemide and sildenafil retained their chemical integrity post-production. DSC thermograms and X-ray diffraction profiles confirmed that the APIs were molecularly dispersed within the polymer matrices, forming amorphous solid dispersions (ASDs) that improved dissolution rates.
From a performance standpoint, dissolution studies demonstrated predictable, reproducible release profiles consistent with the designed architectures. The furosemide-loaded compartments achieved rapid disintegration, while sildenafil’s domain provided controlled, sustained release — validating the structural and chemical rationale of the design.
Mechanical testing further confirmed that the tablets maintained sufficient hardness and friability for handling and transport, despite their internal porosity — a delicate balance rarely achieved in early 3D printed dosage forms.
These findings underscore that precision in manufacturing does not have to compromise robustness. When guided by analytical validation, 3D printed pharmaceuticals can meet — and potentially exceed — traditional GMP standards.
Digital Pharmaceutical Manufacturing: The Dawn of a New Workflow
The adoption of 3D printing in drug production is more than a technological upgrade; it represents a philosophical shift from mass production to mass customization. Instead of central factories producing millions of identical tablets, future pharmaceutical ecosystems could consist of regional or hospital-based micro-factories equipped with certified printers and validated digital blueprints.
This model would enable on-demand manufacturing, dose adjustment, and rapid response to supply chain disruptions. For chronic disease management, 3D printing could allow adaptive dosing protocols — for instance, adjusting diuretic or vasodilator ratios weekly based on patient monitoring data.
Digitization also democratizes formulation science. A single digital file can define geometry, composition, and drug distribution, ensuring reproducibility without re-engineering. Regulatory authorities could certify these digital templates as “virtual formulations,” drastically reducing the need for repetitive approval cycles when only minor adjustments are made for patient personalization.
The implications for sustainability are equally profound. 3D printing minimizes material waste, energy consumption, and transportation needs, aligning pharmaceutical production with global green manufacturing goals.
Challenges and Limitations: The Road Still Under Construction
While the promise of 3D printing in pharmaceuticals is compelling, the road to industrial adoption is lined with challenges — technological, regulatory, and ethical.
First, print resolution and process speed remain limiting factors. Producing individualized tablets is feasible at small scale but not yet competitive with the throughput of rotary tablet presses, which can produce thousands per minute. Efforts are ongoing to develop multi-nozzle printers and parallel extrusion systems to bridge this gap.
Second, the regulatory framework for additive manufacturing is still in its infancy. The U.S. FDA approved the first 3D printed drug, Spritam® (levetiracetam), in 2015, but comprehensive GMP standards for additive manufacturing remain under development. The validation of each printing process, raw material, and software algorithm requires a new level of regulatory sophistication.
Third, real-time release testing (RTRT) — while conceptually feasible with spectroscopic analytics — must meet rigorous reproducibility and traceability standards. The integration of machine learning for anomaly detection and AI-driven feedback loops could soon enable fully autonomous QC systems, but regulatory acceptance will take time.
Lastly, ethical and cybersecurity concerns must be addressed. When drug formulations become digital files, the potential for unauthorized modification, data corruption, or counterfeiting increases. Blockchain-enabled traceability and encrypted prescription-to-production systems will be vital safeguards in this new era.
The Case for Hybrid Manufacturing: Bridging Old and New Worlds
The near-term solution likely lies not in replacing traditional manufacturing but in hybridizing it. 3D printing can complement conventional processes by:
- Producing specialized dosage forms (e.g., pediatric, geriatric, or orphan drug formulations) that lack commercial viability at scale.
- Enabling rapid prototyping of new formulations during R&D, accelerating preclinical development cycles.
- Customizing multi-layered release systems or spatially complex tablets impossible to compress mechanically.
By combining traditional granulation with additive layering or by integrating printed substructures within compressed tablets, manufacturers can harness the best of both worlds — industrial scalability and digital flexibility.
This modular approach could gradually introduce 3D printing into GMP workflows without disrupting established supply chains or validation systems.
The Future of Quality Control: Toward Predictive and Adaptive Systems
The most transformative impact of 3D printing may ultimately lie not in production but in how quality is defined and assured.
Traditional QC is retrospective — test samples after production, compare to specification, release or reject. Additive manufacturing, however, lends itself to predictive QC, where real-time sensor data, process analytics, and digital twins of each tablet create an unbroken chain of quality assurance from blueprint to final dose.
Imagine a manufacturing environment where every tablet’s spectral fingerprint, geometry, and density profile are archived in a cloud-based ledger, verifiable at any point in its lifecycle. Regulatory authorities could access this data remotely, reducing the need for physical inspections. The concept transforms “batch release” into unit release, enhancing transparency, traceability, and trust.
Such integration of AI-driven predictive analytics and IoT-enabled process control will redefine what it means for a medicine to be “quality assured.” The transition may be complex, but the direction is irreversible.
Conclusion
The study “3D printed furosemide and sildenafil tablets: Innovative production and quality control” is far more than a technical demonstration — it is a declaration of intent for the pharmaceutical future. It proves that additive manufacturing can produce chemically stable, structurally precise, and analytically validated dosage forms that rival, and in some respects surpass, conventionally made tablets.
Furosemide and sildenafil served not just as drugs but as symbols — one representing traditional, high-turnover pharmacotherapy; the other representing precision-targeted vasomodulation. Their successful co-printing represents the convergence of two worlds: the reliability of established pharmacology and the flexibility of digital design.
3D printing is not a passing novelty; it is the architectural reinvention of pharmaceutical manufacturing. It aligns perfectly with the ethos of modern medicine — personalization, sustainability, and data-driven precision. The journey from experimental printer to clinical pharmacy may still be long, but its trajectory is unmistakable.
FAQ: 3D Printing and Pharmaceutical Innovation
1. Why are furosemide and sildenafil ideal for 3D printing studies?
They represent two contrasting physicochemical profiles — hydrophilic and hydrophobic, thermally sensitive and stable — providing a comprehensive test of the technology’s adaptability across diverse drug types.
2. How does 3D printing improve tablet quality control?
It enables real-time, non-destructive analysis of each tablet’s structure and composition using NIR spectroscopy, chemometrics, and micro-CT, moving quality control from sampling-based to individualized verification.
3. Will 3D printing replace conventional tablet manufacturing?
Not entirely. It will complement it — serving high-value applications such as personalized therapy, rapid prototyping, or localized manufacturing, while traditional methods continue to dominate large-scale production.