Vein grafting is a cornerstone of modern vascular and cardiac surgery. Whether restoring coronary flow in bypass surgery or repairing peripheral circulation, the autologous vein graft remains a trusted surgical ally. Yet this ally has a critical flaw: its long-term survival is undermined by intimal hyperplasia (IH)—a pathological thickening of the vessel wall that narrows the lumen and leads to graft failure. Despite decades of surgical refinement and pharmacologic innovation, no therapy has effectively eliminated this vexing process.
In an era where nanotechnology is redefining medical paradigms, a group of researchers has developed an elegant, biologically inspired strategy: platelet membrane–coated PLGA nanoparticles loaded with sildenafil citrate. This innovation, combining vascular biology, pharmacology, and nanomedicine, offers a glimpse into a future where drug delivery is not only localized but also biomimetic—using nature’s own targeting machinery to prevent vascular disease at its source.
The Clinical Challenge of Vein Graft Intimal Hyperplasia
When a vein is transplanted into the high-pressure arterial circulation, it undergoes a dramatic physiological transformation. The sudden increase in mechanical stress, shear forces, and oxygen tension triggers endothelial injury and smooth muscle activation. These events ignite a cascade of inflammation, proliferation, and extracellular matrix deposition—collectively known as intimal hyperplasia.
Histologically, IH is characterized by smooth muscle cell migration from the media to the intima, where they proliferate and produce collagen and proteoglycans. Functionally, it behaves like an early form of restenosis, narrowing the vessel lumen and impairing flow. Within one year, up to 50% of vein grafts show significant intimal thickening, and as many as 20% fail clinically due to occlusion or thrombosis.
Clinicians have attempted to mitigate IH using systemic pharmacotherapies—antiplatelets, statins, ACE inhibitors, and even antiproliferative drugs—but the problem persists. The limitation is fundamental: these drugs circulate systemically and fail to accumulate sufficiently at the vascular injury site, where they are needed most. Moreover, systemic delivery increases adverse effects and limits therapeutic dosing.
The need, therefore, is clear: a targeted, local therapy capable of delivering anti-proliferative agents directly to the graft, maintaining therapeutic concentrations over time while sparing the rest of the body. This is precisely where the platelet membrane–encapsulated sildenafil nanoparticles enter the clinical imagination.
Sildenafil: An Old Drug with a New Vascular Role
Sildenafil citrate is best known for its ability to treat erectile dysfunction by inhibiting phosphodiesterase type 5 (PDE5) and augmenting the nitric oxide (NO)–cGMP pathway. However, in vascular biology, its benefits extend far beyond smooth muscle relaxation. By increasing intracellular cGMP, sildenafil inhibits smooth muscle proliferation, reduces inflammatory cytokine production, and enhances endothelial repair—properties that make it a potent anti-intimal agent.
In the context of vein grafts, where smooth muscle overactivation drives neointimal formation, sildenafil’s antiproliferative and vasoprotective effects are highly desirable. Yet systemic sildenafil therapy faces two insurmountable barriers: poor tissue targeting and a short plasma half-life (3–6 hours). High doses would be required to achieve therapeutic concentrations in the graft wall, posing systemic risks such as hypotension and vision disturbances.
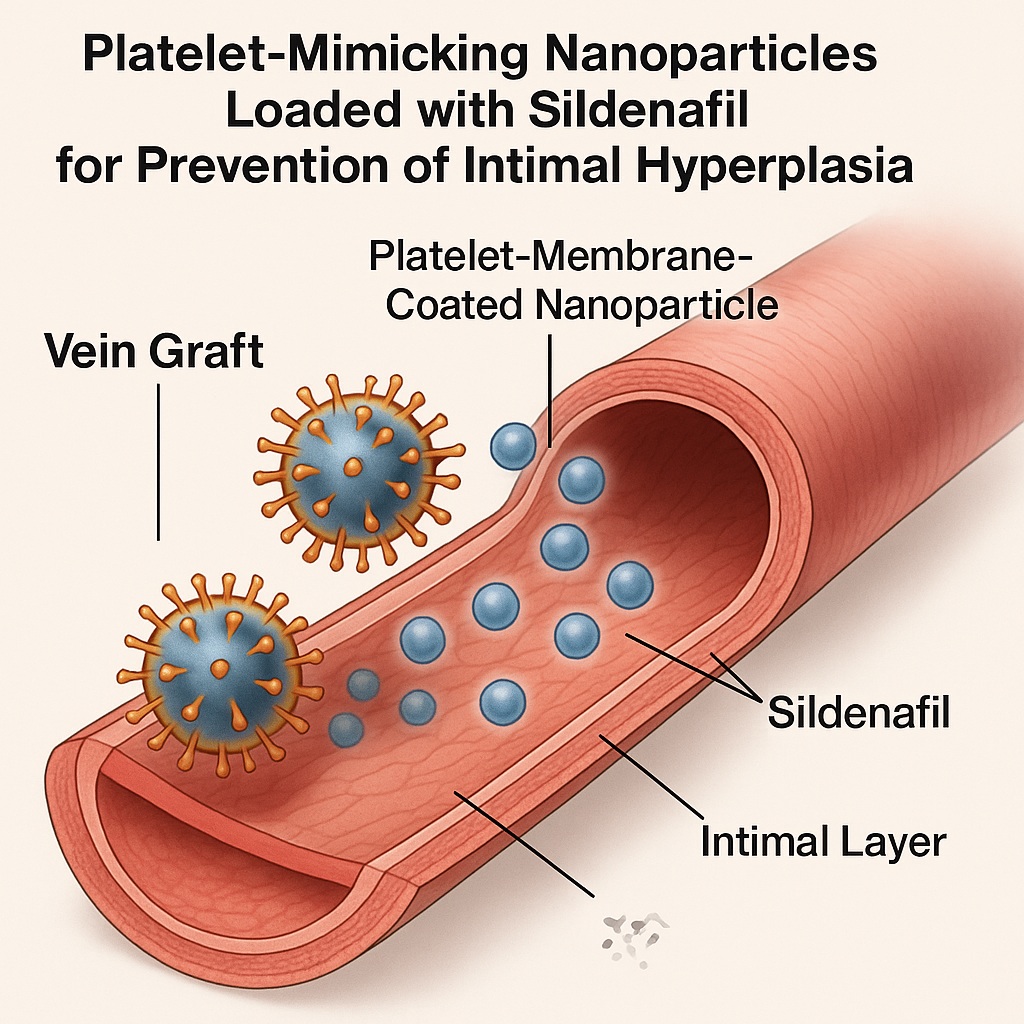
Encapsulating sildenafil in poly(lactic-co-glycolic acid) (PLGA) nanoparticles, and further coating them with platelet membranes, solves both problems elegantly. The approach allows controlled, sustained release and biomimetic targeting, aligning pharmacokinetics with the biological timeline of vascular repair.
Platelet-Membrane Coating: Nature’s Own Targeting System
Platelets are natural first responders to vascular injury. Their membranes are rich in surface adhesion molecules such as P-selectin, CD41, and CD47, which enable them to recognize and bind to exposed collagen, damaged endothelium, and inflammatory cells. By cloaking nanoparticles in platelet-derived membranes, scientists effectively bestow these targeting abilities onto a synthetic drug carrier.
This biomimetic camouflage serves multiple purposes:
- Immune evasion, as the body recognizes the membrane as self and avoids clearance by macrophages.
- Targeted adhesion to the injury site, driven by platelet surface ligands that interact with activated endothelium and inflammatory foci.
- Prolonged circulation time, allowing sustained presence in the bloodstream and gradual drug release.
The result is a nanoparticle that behaves like a living cell fragment: circulating harmlessly until it encounters vascular injury, where it adheres and delivers its pharmacologic payload precisely where it is needed. In preclinical models, this approach has achieved selective accumulation in grafted vessels, significantly enhancing local drug concentration while minimizing systemic exposure.
PLGA Nanoparticles: The Engine of Sustained Drug Release
PLGA (poly(lactic-co-glycolic acid)) is a biodegradable, FDA-approved polymer widely used for controlled drug delivery. Its degradation into lactic and glycolic acids allows safe elimination through natural metabolic pathways. More importantly, by modulating the ratio of lactic to glycolic components, scientists can fine-tune drug release kinetics—from hours to weeks.
In the platelet-membrane-coated system, PLGA forms the core nanoparticle encapsulating sildenafil. Upon adhesion to the injured vessel wall, the polymer slowly degrades, releasing sildenafil in a sustained and predictable manner. This sustained exposure aligns with the pathophysiologic timeline of intimal hyperplasia, which develops gradually over days to weeks following graft implantation.
Such temporal synchronization between drug release and disease progression is one of nanomedicine’s most transformative features. Instead of transient pharmacologic spikes, the system ensures stable, localized exposure, maximizing efficacy while reducing toxicity.
In Vivo Evidence: A Promising Preclinical Reality
In experimental vein graft models, the platelet membrane–encapsulated sildenafil nanoparticles demonstrated compelling results. Compared with free sildenafil or uncoated nanoparticles, the biomimetic formulation achieved:
- Higher accumulation in the grafted vein segments, confirmed by fluorescence tracking,
- Sustained sildenafil release over several days,
- Significant reduction in neointimal thickness and smooth muscle proliferation,
- Improved endothelial continuity and reduced inflammatory infiltration.
Histological sections from treated grafts revealed preserved luminal architecture and decreased collagen deposition—hallmarks of successful IH prevention. Importantly, systemic sildenafil levels remained low, and no adverse hemodynamic or hepatic effects were detected, underscoring the platform’s safety and pharmacologic efficiency.
The translational message is clear: this strategy achieves what systemic drugs cannot—targeted vascular therapy that acts locally but spares the rest of the organism.
Clinical Translation: From Bench to Bypass Surgery
For vascular surgeons and interventional cardiologists, the clinical potential of this technology is enormous. Current postoperative management of vein grafts relies on antiplatelet agents, statins, and risk-factor modification. Yet none of these directly addresses the smooth muscle hyperplasia that drives restenosis.
A sustained-release, locally targeted therapy capable of dampening vascular proliferation could become a cornerstone adjunct to grafting procedures. The delivery platform described here offers multiple clinically relevant advantages:
- Compatibility with existing surgical workflows: Nanoparticles can be administered perioperatively via local infusion into the graft lumen or systemically immediately after surgery.
- Reduced systemic side effects: By targeting only the graft site, sildenafil’s vasodilatory and hypotensive risks are minimized.
- Potential for broad adaptation: Beyond vein grafts, similar systems could be applied to stent coatings, arteriovenous fistula maturation, or post-angioplasty restenosis.
For clinicians, such technology represents not only a therapeutic innovation but also a paradigm shift—from systemic pharmacology to precision vascular nanotherapy.
Mechanistic Implications: The Molecular Symphony of Graft Protection
At the molecular level, the efficacy of platelet-coated sildenafil nanoparticles arises from a synergistic triad of mechanisms:
- NO–cGMP Pathway Enhancement: Sildenafil sustains intracellular cGMP levels, suppressing smooth muscle proliferation and migration, both pivotal drivers of IH.
- Anti-inflammatory Modulation: Reduced expression of TNF-α and adhesion molecules mitigates leukocyte recruitment, decreasing chronic vascular inflammation.
- Endothelial Preservation: By stabilizing endothelial cells and promoting re-endothelialization, the therapy restores the vessel’s natural antithrombotic barrier.
The platelet membrane’s targeting further ensures that these molecular effects occur precisely at the vascular injury interface, where signaling cascades are most dysregulated. This fine spatial control transforms an old drug into a surgical tool of molecular precision.
Safety, Regulatory, and Ethical Considerations
While preclinical data are promising, clinical translation demands rigorous safety evaluation. The biocompatibility of both PLGA and platelet membranes is well established, but batch variability, membrane sourcing, and immunogenicity must be tightly controlled.
Autologous platelet membranes offer an elegant solution, minimizing immune rejection, but their preparation must be standardized for surgical workflows. Furthermore, scaling nanoparticle production under Good Manufacturing Practice (GMP) conditions remains a logistical challenge.
From a regulatory standpoint, this therapy falls within the emerging category of combination biologic-nanomedicine products, requiring oversight from both pharmacologic and medical device authorities. However, the clinical need it addresses—vein graft failure—carries such significant morbidity that the risk-benefit balance may favor early translational trials.
Ethically, the use of platelet-derived materials raises no major objections, provided donors are consented and screened. Indeed, this bio-inspired approach exemplifies the convergence of biotechnology and surgery in a way that enhances safety rather than complicates it.
Looking Ahead: The Future of Personalized Vascular Therapy
The convergence of nanotechnology, biomimicry, and vascular surgery is ushering in a new era of targeted postoperative care. In the near future, a patient undergoing coronary artery bypass might receive a customized nanotherapeutic infusion tailored to their graft type, risk profile, and comorbidities.
Beyond sildenafil, the platelet membrane–PLGA platform could be adapted to deliver other agents—sirolimus, nitric oxide donors, anti-inflammatory cytokines, or genetic material—all precisely localized to the graft site. Combined with intraoperative imaging and vascular mapping, such personalized regimens could dramatically reduce graft loss, repeat surgeries, and long-term complications.
Clinicians should view this not as speculative science but as a logical evolution. Just as drug-eluting stents revolutionized interventional cardiology, biomimetic, drug-loaded nanoparticles may soon redefine the standard of vascular reconstruction and graft maintenance.
Conclusion: A Therapeutic Concept Whose Time Has Come
Intimal hyperplasia has long been an unavoidable adversary in vascular grafting. The development of platelet membrane–encapsulated sildenafil nanoparticles represents a sophisticated and clinically sensible approach to overcoming this challenge. By combining targeted adhesion, sustained drug release, and physiological biocompatibility, this platform addresses the root mechanisms of graft failure—smooth muscle proliferation, inflammation, and endothelial dysfunction.
For practicing physicians, the message is pragmatic: the future of vascular medicine is moving toward site-specific, self-guided therapies that act where pathology begins. This innovation is more than a pharmacologic improvement—it is the embodiment of precision medicine at the vascular interface.
FAQ: Platelet-Coated Sildenafil Nanoparticles in Vascular Therapy
1. How do platelet-mimicking nanoparticles differ from traditional drug-eluting systems?
Unlike polymer-coated stents or local infusions, platelet-coated nanoparticles actively seek out sites of vascular injury using natural adhesion molecules, ensuring drug delivery is both targeted and dynamic.
2. Could this therapy be applied beyond vein grafts?
Yes. The same principle could benefit arterial bypasses, arteriovenous fistulas in dialysis patients, and even post-angioplasty restenosis, wherever intimal hyperplasia limits long-term patency.
3. When might this technology reach clinical trials?
Preclinical studies show strong safety and efficacy data. With appropriate GMP-scale production and regulatory approval, early-phase clinical trials could begin within the next few years, particularly in high-risk vascular surgery populations.