Introduction
Ureteral stents are among the most frequently used devices in modern urology. They are indispensable in restoring urine flow, preserving renal function, and facilitating healing after ureteral surgery. Yet, despite their benefits, they have one notorious downside: stent-related discomfort (SRD). Patients often describe it as a relentless combination of urinary urgency, frequency, flank pain, and a disconcerting awareness that something is constantly “out of place.”
Studies report that up to 88% of patients with stents experience some form of SRD, and more than 70% resort to analgesics for relief. The result is not just physical suffering but also compromised quality of life, diminished work efficiency, and emotional frustration. Ironically, a device designed to protect renal health becomes a source of morbidity. Unsurprisingly, clinicians and researchers have been searching for pharmacological solutions to this problem.
The latest systematic review and meta-analysis from the Korean Society of Endourology and Robotics (KSER) provides a comprehensive evaluation of four main pharmacological classes for alleviating SRD: alpha-blockers, antimuscarinics, beta-3 agonists, and phosphodiesterase-5 (PDE5) inhibitors. Their work synthesizes evidence from 16 randomized controlled trials (RCTs), covering 1,865 patients, to clarify which medications genuinely improve symptoms and which merely shine in theory.
Understanding Stent-Related Discomfort
Stent-related discomfort remains frustratingly complex. The exact mechanisms remain elusive, but several overlapping hypotheses exist:
- Smooth muscle spasm in the ureter and bladder may cause pain and urgency.
- Mechanical irritation of the trigone or bladder mucosa by the stent contributes to frequency and urgency.
- Reflux and pressure transmission during voiding can create flank pain and discomfort.
- Stent migration and movement may lead to additional irritation.
Importantly, not all symptoms are equal. Pain tends to respond better to certain therapies than urinary frequency or urgency. This discrepancy suggests different underlying mechanisms for each symptom domain, making SRD management a true therapeutic puzzle.
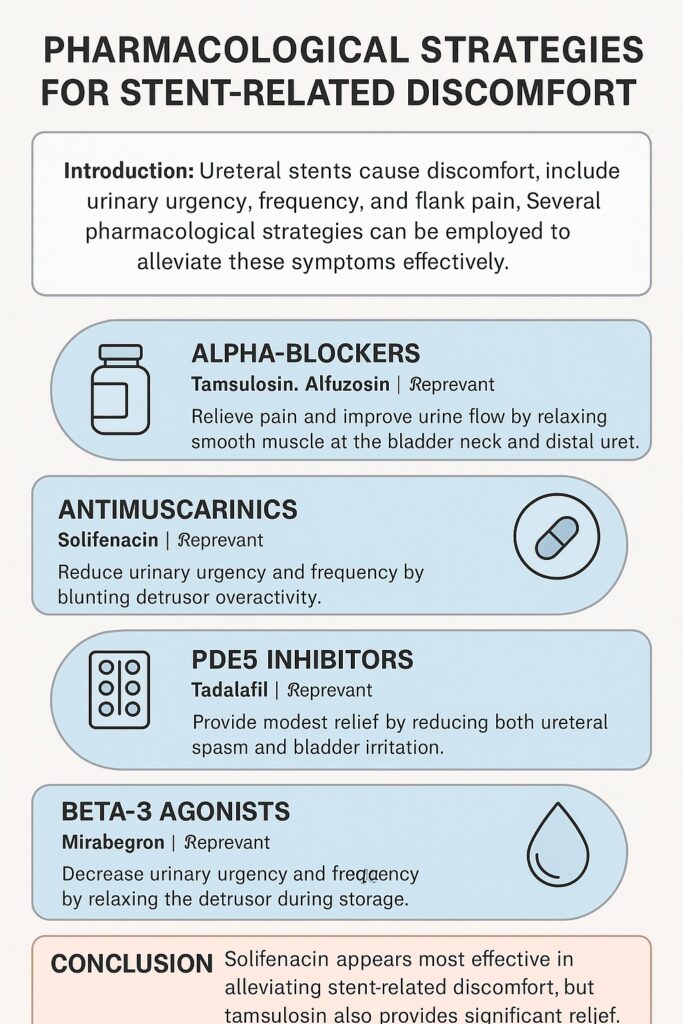
Alpha-Blockers: The Traditional Workhorse
Alpha-blockers such as tamsulosin, alfuzosin, and silodosin have long been the frontline agents in SRD management. Their mechanism lies in relaxing smooth muscle at the bladder neck and distal ureter, thereby reducing spasm and improving urine flow.
The KSER meta-analysis confirmed that alpha-blockers significantly reduced urinary symptom scores (USS) compared to placebo. Notably:
- Tamsulosin consistently demonstrated strong efficacy, often outperforming alfuzosin.
- Subtype selectivity matters: tamsulosin’s dual affinity for alpha-1a and alpha-1d receptors may explain its superiority over non-selective alfuzosin.
- Silodosin, despite theoretical advantages due to its receptor profile, failed to achieve significant superiority over placebo in pooled analysis.
Clinical interpretation is straightforward: tamsulosin remains the best-supported alpha-blocker for SRD. Alfuzosin is not far behind but may offer less consistent relief.
Antimuscarinics: Targeting Irritative Symptoms
Solifenacin, a selective antimuscarinic, has been tested for its ability to reduce stent-induced urgency and frequency. Antimuscarinics blunt detrusor overactivity, which seems particularly relevant when bladder irritation dominates the clinical picture.
The evidence is compelling. The KSER review showed that solifenacin achieved the highest P-score (0.8484) in ranking probability analysis, meaning it outperformed other agents in terms of urinary symptom control. Compared to placebo, solifenacin produced the largest reduction in USS, suggesting it is the most effective monotherapy currently available.
An interesting nuance emerges: solifenacin does not necessarily reduce pain as effectively as alpha-blockers. Instead, its strength lies in tackling irritative urinary symptoms such as frequency and urgency. This makes it a valuable tool, particularly for patients who describe their primary burden as “constant need to urinate” rather than pain.
Beta-3 Agonists: A Newcomer with Modest Promise
Mirabegron, a beta-3 agonist, relaxes the detrusor muscle during the storage phase of micturition. It has already proven its worth in treating overactive bladder. But can it help patients with a foreign object irritating the urinary tract?
The answer, at least for now, is mixed. Some individual trials suggested mirabegron improves urgency and frequency. However, pooled meta-analysis showed no statistically significant advantage over placebo. While the mean difference trended in the right direction, confidence intervals crossed zero, indicating uncertainty.
Still, mirabegron remains attractive due to its favorable side-effect profile compared to antimuscarinics. In practice, it may benefit patients intolerant of solifenacin. Yet, clinicians should temper expectations—it is not the silver bullet some hoped for.
PDE5 Inhibitors: A Surprising Contender
Tadalafil, best known for erectile dysfunction and lower urinary tract symptoms (LUTS), emerged as an unexpected candidate for SRD management. PDE5 inhibitors enhance smooth muscle relaxation and improve blood flow, mechanisms that could plausibly reduce both ureteral spasm and bladder irritation.
The KSER review demonstrated that tadalafil reduced USS compared with placebo, albeit modestly (mean difference: −2.96). While the effect was statistically significant in pairwise analysis, the benefit diminished in network comparisons, suggesting that tadalafil is helpful but not as potent as solifenacin or tamsulosin.
Nevertheless, tadalafil may be particularly appealing for men with concomitant erectile dysfunction, offering a “two birds with one stone” solution.
Comparative Effectiveness: The League Table
When medications were ranked head-to-head in Bayesian network analysis, the hierarchy became clear:
- Solifenacin – most effective for overall USS reduction.
- Tamsulosin – highly effective, particularly for pain relief.
- Alfuzosin – helpful but less potent than tamsulosin.
- Tadalafil – modest benefit, with niche utility.
- Mirabegron – safe, but evidence remains inconclusive.
- Silodosin – did not outperform placebo.
This ranking supports a pragmatic approach: start with solifenacin or tamsulosin, tailor therapy based on dominant symptoms, and consider tadalafil or mirabegron in select cases.
Beyond Medication: The Role of Stent Design
While pharmacology dominates the conversation, mechanical factors matter too. Stent material, diameter, length, and tip design all influence patient comfort. Unfortunately, trials have yielded inconsistent results. Softer stents sometimes improve symptoms, but not always. Shorter stents reduce urgency but risk migration. In the end, even the most perfectly engineered stent is still a foreign body—and discomfort is nearly inevitable.
Thus, medications remain essential adjuncts, not replacements for thoughtful stent design.
Clinical Implications
The implications of this meta-analysis extend beyond academic interest. In daily practice, clinicians face patients who are less interested in receptor subtypes and more concerned with whether they can sleep through the night without running to the bathroom.
A practical clinical pathway might look like this:
- Pain-predominant SRD → Tamsulosin.
- Urgency/frequency-predominant SRD → Solifenacin.
- Mixed symptoms → Combination therapy (tamsulosin + solifenacin has shown additive effects in other studies).
- Patients with erectile dysfunction → Tadalafil as a dual-purpose therapy.
- Solifenacin intolerance or contraindication → Consider mirabegron.
Such a tailored strategy acknowledges the multifactorial nature of SRD while grounding choices in evidence.
Limitations and Future Directions
No meta-analysis is without limitations. This one, despite its rigor, had several:
- Variability in medication doses and outcome measures across trials.
- Limited data on side effects, which are crucial in selecting long-term therapies.
- Lack of stratification by stent characteristics (material, size, length).
- Inconsistent reporting of quality-of-life endpoints beyond the USSQ.
Future research must address these gaps, particularly head-to-head trials comparing solifenacin and tamsulosin, as well as combination therapies. There is also growing interest in biodegradable stents, which might one day render pharmacological management less necessary.
Conclusion
Stent-related discomfort remains an unfortunate byproduct of a highly useful medical device. Fortunately, pharmacology offers several tools to mitigate this burden.
- Solifenacin emerges as the most effective agent for reducing urinary symptom burden.
- Tamsulosin remains highly effective, especially for pain.
- Alfuzosin and tadalafil provide modest relief.
- Mirabegron is safe but less proven, and silodosin disappoints.
For clinicians, the key is not choosing a universal best drug but tailoring therapy to the patient’s dominant symptoms and comorbidities. For patients, the message is simple: while stents will never be comfortable, they no longer need to be unbearable.
FAQ
1. Which medication is most effective for stent-related discomfort?
Current evidence points to solifenacin as the most effective agent, especially for irritative urinary symptoms such as urgency and frequency.
2. Should every patient with a ureteral stent receive medication?
Not necessarily. Mild cases may tolerate stents without intervention. However, in patients reporting significant discomfort, initiating therapy—usually with tamsulosin or solifenacin—is strongly recommended.
3. Can combination therapy be used?
Yes. Combining an alpha-blocker (for pain and spasm) with an antimuscarinic (for urgency and frequency) often provides additive benefits. This approach is reasonable when monotherapy fails.


