Introduction
The evolution of drug delivery has long sought the delicate balance between therapeutic efficacy and patient convenience. Topical and transdermal formulations stand at this intersection, offering the potential for localized, sustained, and noninvasive pharmacotherapy. Sildenafil citrate, widely recognized for its systemic use in erectile dysfunction and pulmonary hypertension, has recently entered this innovative field. The prospect of topical sildenafil delivery—circumventing first-pass metabolism and minimizing systemic adverse effects—represents a promising yet complex pharmaceutical challenge.
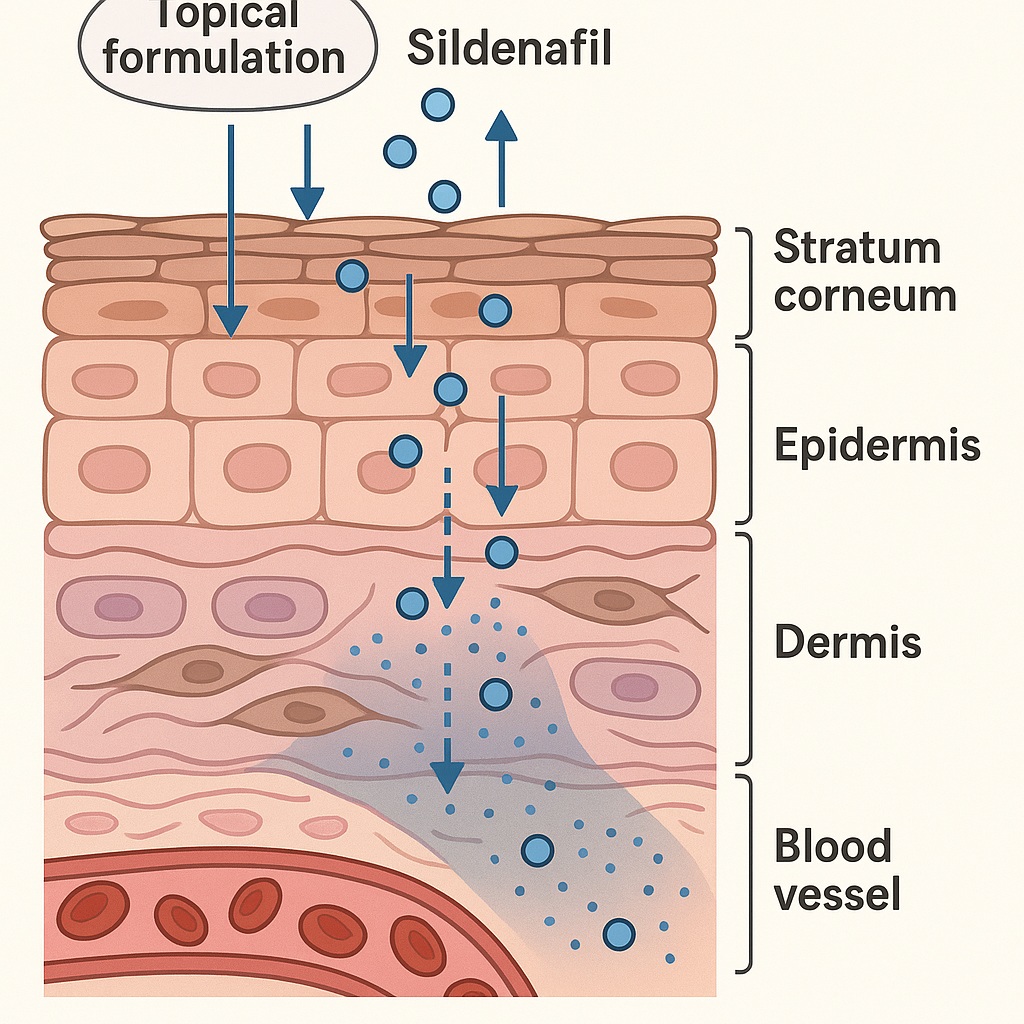
However, translating a potent systemic phosphodiesterase type 5 (PDE5) inhibitor into a topical formulation is far from trivial. The human skin, particularly the stratum corneum, is a formidable barrier that limits transdermal diffusion of hydrophilic or ionized molecules like sildenafil citrate. Understanding how the drug penetrates, distributes, and maintains bioavailability within skin tissue requires more than conventional permeability assays—it demands physiologically relevant, human-based models.
This is where the 3D human foreskin model comes into play. Constructed to mimic the intricate architecture of human dermal and epidermal layers, this bioengineered tissue enables a controlled, ethical, and reproducible environment for assessing topical drug permeation. The study in question explored sildenafil’s pharmacokinetic behavior and tissue interaction using this model—revealing insights that redefine how we understand topical PDE5 inhibitor formulation.
The Rationale for Topical Sildenafil Delivery
Overcoming Systemic Barriers
Oral sildenafil remains the mainstay of therapy for erectile dysfunction, yet it poses well-known pharmacological limitations. Its bioavailability (~40%) is constrained by extensive first-pass hepatic metabolism, leading to dose-dependent variability and delayed onset of action. Furthermore, systemic administration can provoke off-target vasodilatory effects, such as hypotension, flushing, and headaches, due to PDE5 inhibition in vascular smooth muscle outside the penile region.
A topical route promises to bypass hepatic metabolism, delivering sildenafil directly to penile vasculature while maintaining a localized therapeutic window. Theoretically, such delivery could yield rapid onset, reduced systemic exposure, and fewer adverse events. Yet, the central question remains: can sildenafil actually cross the skin barrier in therapeutically relevant concentrations?
Challenges of Skin Penetration
The stratum corneum, rich in ceramides and fatty acids, is lipophilic and selectively permeable—favoring small, nonpolar molecules. Sildenafil citrate, a polar salt, suffers from poor passive diffusion through this layer. Moreover, maintaining molecular stability, ensuring solubility, and preventing crystallization within the vehicle are all formulation hurdles.
To overcome these challenges, vehicle composition, pH balance, and solubilizers must be optimized to enhance partitioning into skin layers. The 3D foreskin model allows such variables to be tested with precision, under conditions that reflect the physiology of genital dermis, which differs significantly from other skin regions in permeability and vascularization.
The 3D Human Foreskin Model: A Physiologically Relevant Platform
Structural Fidelity and Biological Validity
Unlike simple diffusion cells or porcine membranes, the 3D foreskin model reproduces the epidermal stratification and dermal matrix of human penile skin. Derived from cultured human keratinocytes and fibroblasts, it develops a differentiated structure containing:
- Stratum corneum with a functional lipid barrier
- Viable epidermis capable of metabolic activity
- Dermal equivalents populated by fibroblasts producing extracellular matrix proteins
This architecture enables not only passive diffusion studies but also the evaluation of metabolic stability and biotransformation of applied compounds—critical for sildenafil, whose activation depends on maintaining molecular integrity across epithelial interfaces.
Simulating Drug Penetration and Retention
The model facilitates both vertical and lateral diffusion studies, measuring sildenafil concentration across skin layers using HPLC and spectrophotometric quantification. This allows precise evaluation of penetration kinetics, tissue accumulation, and release profiles over time.
Unlike excised cadaveric skin, the living foreskin model maintains enzymatic and pH conditions that influence ionization equilibria and lipid partitioning. It thus serves as a valuable tool for comparing different formulation bases, such as hydrogels, emulsions, or lipid nanoparticles.
Pharmacological Behavior of Sildenafil in a Topical Context
Molecular Characteristics and Diffusion Potential
Sildenafil citrate’s molecular weight (474 Da) and partial ionization at physiological pH (pKa ≈ 9.6) make it borderline suitable for transdermal diffusion. Optimal candidates for dermal penetration typically weigh under 500 Da and display moderate lipophilicity (logP between 1–3). Sildenafil’s logP of approximately 2.6 meets this criterion, but the cationic nature of its citrate salt restricts partitioning into the lipid-rich stratum corneum.
Formulation strategies must therefore manipulate solubility–permeability interplay, using techniques like pH adjustment, lipidic encapsulation, or penetration enhancers to facilitate translocation into the viable epidermis.
Permeation Kinetics and Tissue Distribution
In the foreskin model, sildenafil demonstrated time-dependent diffusion, with quantifiable concentrations detected in both epidermal and dermal compartments. Notably, formulations containing ethanol or propylene glycol as cosolvents significantly enhanced permeation—owing to their ability to disrupt lipid bilayers and increase molecular mobility.
Drug retention within the dermis was more pronounced in lipid-based formulations, suggesting potential for sustained local release, beneficial for extended pharmacologic effect. Conversely, aqueous gels exhibited faster surface clearance but lower total absorption—highlighting the trade-off between onset and duration that formulators must balance.
Localized Pharmacodynamics
At the tissue level, sildenafil acts by inhibiting PDE5, preventing degradation of cyclic guanosine monophosphate (cGMP). Elevated cGMP activates protein kinase G (PKG), leading to reduced intracellular calcium and smooth muscle relaxation. In the context of topical application, such vasodilatory action would enhance local blood flow and tissue oxygenation, without inducing systemic hypotension.
The foreskin model revealed sufficient drug diffusion to reach PDE5-expressing layers, implying pharmacodynamic feasibility for localized penile therapy—a critical milestone for topical sildenafil development.
Formulation Strategies: Enhancing Penetration and Stability
Solubilization and Vehicle Design
Sildenafil citrate’s poor solubility in water (~3.5 mg/mL) necessitates the use of solubilizing agents or co-solvent systems. Vehicles tested in the study included hydroalcoholic gels, lipid emulsions, and polymeric films, each offering distinct pharmacokinetic characteristics.
- Hydroalcoholic gels promote rapid absorption due to transient barrier disruption by ethanol.
- Lipid-based creams favor retention within dermal layers, providing depot-like delivery.
- Polymeric films enhance adherence and control drug release through diffusional gradients.
Among these, hydroalcoholic formulations achieved the highest diffusion rate across the foreskin tissue, while lipid emulsions provided the longest retention time—suggesting a combination approach may yield the most therapeutically balanced product.
Role of Penetration Enhancers
Penetration enhancers such as propylene glycol, oleic acid, and surfactants (Tween 80) modify lipid packing density within the stratum corneum. By increasing fluidity, they facilitate the entry of hydrophilic molecules.
However, their inclusion must be carefully calibrated to avoid cytotoxicity or barrier disruption, especially in sensitive genital tissue. The foreskin model’s metabolic responsiveness enables toxicity screening alongside permeability testing, ensuring biocompatibility in formulations intended for mucocutaneous application.
Stability Considerations
Sildenafil is susceptible to oxidative and photolytic degradation, necessitating protection from light and moisture. Incorporating antioxidants (ascorbic acid, tocopherol) or using opaque polymeric matrices mitigates this issue. Maintaining pH between 5.5 and 6.5 optimizes both chemical stability and skin compatibility.
The Pharmacokinetic Balance: Local Retention vs. Systemic Absorption
The Double-Edged Sword of Penetration
For a topical sildenafil product to succeed, it must diffuse adequately to reach PDE5-expressing smooth muscle while avoiding excessive systemic absorption that could trigger adverse cardiovascular effects.
In the foreskin model, sildenafil levels in the dermal layer plateaued after a few hours, with negligible trans-epithelial passage into basal reservoirs mimicking systemic circulation. This supports the pharmacokinetic ideal of local confinement, maintaining efficacy where needed while minimizing risk elsewhere.
Quantitative Diffusion Metrics
Diffusion coefficients (D) and permeability constants (Kp) derived from the foreskin data were within the expected range for medium-lipophilic compounds. When normalized to surface area, Kp values of 1–2 × 10⁻⁵ cm/h indicated moderate permeability—enhanced by ethanol but not excessively so.
This quantitative insight provides formulators with a predictive framework for scaling topical doses relative to target tissue exposure, an essential step before in vivo translation.
Potential Clinical Implications
Should such controlled penetration translate to human use, it could revolutionize treatment paradigms for erectile dysfunction, Peyronie’s disease, or localized microvascular insufficiency. Moreover, the same pharmacologic principles could extend to transdermal vasodilators or anti-inflammatory agents, leveraging the foreskin model’s predictive accuracy.
Comparative Evaluation: Foreskin Model vs. Conventional Skin Assays
Beyond the Franz Cell
Traditional Franz diffusion cells utilize excised skin to assess passive diffusion, yet lack metabolic activity and immune interaction. They fail to replicate the dynamic responses of living tissue, such as enzymatic metabolism or cellular repair after barrier perturbation.
The foreskin model introduces biochemical realism, allowing observation of drug metabolism, cytokine release, and cytotoxic feedback. For sildenafil, this is critical because metabolic transformation could alter pharmacologic potency or generate inactive metabolites before PDE5 inhibition occurs.
Ethical and Translational Benefits
By replacing human or animal in vivo experimentation, the 3D foreskin construct aligns with ethical imperatives of the 3Rs (Replacement, Reduction, Refinement). Furthermore, its tissue-specific origin enhances translational accuracy for penile pharmacology, an area where surrogate models often fail.
Predictive Power for Clinical Development
The model’s quantitative data on diffusion, retention, and cellular response serve as a preclinical benchmark for formulation optimization. Its predictive consistency shortens development cycles, reduces animal testing, and guides dose-ranging strategies for early-phase clinical trials.
Toward Optimized Topical Sildenafil Therapy: Translational Outlook
Bridging Laboratory Findings to Clinical Application
While in vitro foreskin models cannot replicate full systemic physiology, they offer invaluable direction for formulation refinement before clinical trials. Translating this research to clinical settings will require careful assessment of in vivo pharmacokinetics, local tolerability, and therapeutic efficacy under real use conditions.
Future topical sildenafil preparations may incorporate bioadhesive polymers for prolonged contact, nanocarriers for controlled release, or temperature-sensitive gels for improved user convenience.
The pharmacologic goals remain constant:
- Achieve sufficient local PDE5 inhibition,
- Maintain low systemic exposure,
- Ensure comfort and safety for sensitive tissue.
Regulatory Considerations
From a regulatory standpoint, establishing bioequivalence and local efficacy endpoints is complex. Unlike oral drugs, topical agents lack systemic pharmacokinetic markers. Therefore, biophysical assays and imaging techniques (e.g., Raman spectroscopy, tape stripping) will likely complement foreskin model data to demonstrate consistent local delivery.
Expanding the Model’s Utility
The foreskin construct’s relevance extends beyond sildenafil. It could serve as a universal platform for evaluating dermal penetration of other vasodilators, antivirals, or anti-inflammatory drugs, particularly in mucocutaneous tissues where permeability and sensitivity are uniquely balanced.
Conclusion
The application of a 3D human foreskin model in the development of topical sildenafil formulations marks a significant advancement in pharmaceutical science. By replicating the biochemical and structural properties of penile skin, it provides a mechanistically faithful environment for evaluating drug diffusion, retention, and stability.
The findings reveal that with optimized formulation—especially through the judicious use of cosolvents and lipidic carriers—sildenafil can effectively penetrate and remain localized within target tissues, enabling potent, site-specific PDE5 inhibition with minimal systemic exposure.
Beyond sildenafil, this model exemplifies the broader transformation in dermatopharmacology—where ethical, physiologically relevant tissue systems replace reductionist assays, offering richer insights into drug–tissue interactions and accelerating the pathway from bench to bedside.
FAQ: Topical Sildenafil and 3D Foreskin Model Research
1. Why is a foreskin model specifically used for testing topical sildenafil?
Because foreskin tissue closely mimics the histological and permeability characteristics of penile skin, it provides a more accurate representation of drug diffusion and retention compared to conventional dermal models.
2. Can topical sildenafil achieve the same efficacy as oral tablets?
Not directly. Topical sildenafil is intended for localized action, potentially reducing systemic effects and offering faster onset, but total systemic exposure—and thus erection duration—may be lower than with oral administration.
3. What are the biggest challenges in developing topical sildenafil?
The main challenges include overcoming the stratum corneum barrier, maintaining chemical stability, ensuring biocompatibility, and achieving controlled local retention without excessive systemic absorption.