Introduction
The medical sciences have long struggled with one deceptively simple problem: how to know exactly what is present in a biological system, and in what quantity. Drugs, metabolites, and biochemical markers often share overlapping chemical signatures, making their detection a task that demands both precision and innovation. While traditional chromatographic and spectrophotometric methods have served medicine well, the dawn of nanotechnology has introduced a more elegant solution—nanoparticle-based sensing.
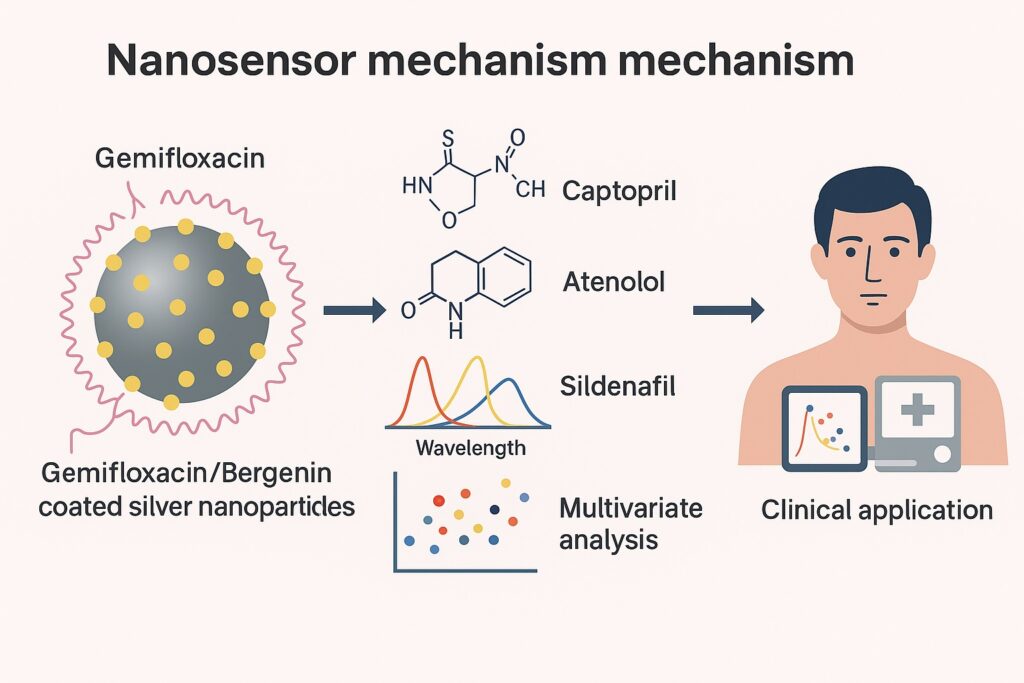
The study under discussion describes a novel nanosensor based on silver nanoparticles (Ag-NPs) coated with Gemifloxacin and Bergenin, which can simultaneously detect captopril (an antihypertensive ACE inhibitor), atenolol (a beta-blocker), and sildenafil citrate (a PDE5 inhibitor widely known for its role in erectile dysfunction therapy). Using advanced multivariate statistical methods, the researchers demonstrated remarkable sensitivity, reproducibility, and selectivity.
In this article, we will explore the broader context and the mechanistic depth of this work. From the pharmacological significance of the three target drugs, to the unique chemical interactions enabling nanosensing, to the statistical methods validating the results, this discussion aims to provide a comprehensive view of how nanotechnology is reshaping the way we monitor pharmacotherapy.
Why Detecting Captopril, Atenolol, and Sildenafil Matters
These three drugs—captopril, atenolol, and sildenafil—may appear to belong to very different therapeutic classes, but they share a unifying theme: cardiovascular relevance.
Captopril, one of the earliest angiotensin-converting enzyme (ACE) inhibitors, remains a cornerstone in managing hypertension and congestive heart failure. It functions by inhibiting the enzymatic conversion of angiotensin I to angiotensin II, thereby reducing vasoconstriction and aldosterone-mediated fluid retention. Precise dosing is crucial, as under-dosing diminishes therapeutic effect, while overdosing risks renal impairment and dangerous hypotension.
Atenolol, a cardioselective beta-1 adrenergic blocker, similarly occupies a vital role in cardiovascular pharmacology. By reducing heart rate and myocardial oxygen demand, it is used to treat hypertension, angina, and arrhythmias. Yet, inappropriate dosing can lead to bradycardia, heart block, or metabolic complications in susceptible individuals.
Sildenafil citrate, although more popularly associated with erectile dysfunction, exerts its therapeutic effect via inhibition of phosphodiesterase type 5 (PDE5), leading to increased nitric oxide-mediated vasodilation. Beyond urology, sildenafil is used in pulmonary arterial hypertension (PAH), where monitoring its bioavailability becomes critical to balancing efficacy and systemic side effects.
Thus, the simultaneous detection of these drugs is not merely an academic exercise—it represents an important step toward polypharmacy monitoring, ensuring safety and efficacy in patients who may receive combinations of these cardiovascular agents.
Silver Nanoparticles: More Than Just Shiny Chemistry
Silver has captivated humans since antiquity, not only for its luster but also for its biological properties. In the nanoscale domain, silver nanoparticles (Ag-NPs) exhibit unique surface plasmon resonance (SPR), a property where conduction electrons oscillate in resonance with incident light. This results in strong and tunable absorption bands in the visible region of the electromagnetic spectrum, which are highly sensitive to changes in the nanoparticle surface environment.
By coating these nanoparticles with specific molecules—such as Gemifloxacin and Bergenin—the researchers effectively created molecular recognition layers. When captopril, atenolol, or sildenafil interacted with the coated nanoparticles, the SPR signals shifted in a manner that could be captured and analyzed via UV–Vis spectroscopy.
The brilliance of this approach lies in its simplicity: instead of complex chromatography or expensive mass spectrometry, the nanosensor relies on the natural physics of electron oscillation at the nanoscale, combined with intelligent surface chemistry.
The Role of Gemifloxacin and Bergenin in Nanosensing
Why Gemifloxacin and Bergenin? At first glance, an antibiotic and a phytochemical may appear unlikely candidates for sensor design. However, their selection is rooted in chemistry.
Gemifloxacin, a fluoroquinolone antibiotic, contains multiple functional groups capable of interacting with both metallic surfaces and pharmaceutical analytes. Its quinolone backbone provides aromaticity, while carboxyl and keto groups allow coordination with silver ions. This dual nature makes it an excellent stabilizer and recognition molecule.
Bergenin, a naturally occurring C-glucoside of 4-O-methyl gallic acid, contributes hydroxyl and lactone groups, enabling hydrogen bonding and additional molecular interactions with analytes. Its antioxidant properties further stabilize the nanoparticles, preventing unwanted aggregation.
Together, Gemifloxacin and Bergenin act as bi-functional capping agents, providing both colloidal stability and specific binding sites. This ensures that when captopril, atenolol, or sildenafil enters the system, the nanoparticles undergo measurable optical changes—essentially transforming them into chemical sentinels.
Multivariate Statistical Tools: From Noise to Knowledge
The challenge with spectroscopic methods lies in interpretation. UV–Vis spectra of complex mixtures often overlap, making it impossible to distinguish signals by naked eye inspection. This is where multivariate statistical tools become indispensable.
The researchers employed methods such as Principal Component Analysis (PCA), Partial Least Squares Discriminant Analysis (PLS-DA), and Partial Least Squares Regression (PLSR).
- PCA reduces the dimensionality of data, highlighting differences between drug-induced spectral changes and background noise.
- PLS-DA allows classification of the analytes, ensuring that captopril, atenolol, and sildenafil could be distinctly recognized despite overlapping signals.
- PLSR quantifies the concentration of each drug with high accuracy, turning spectral shifts into clinically meaningful numbers.
This marriage of nanotechnology with chemometrics exemplifies modern pharmacological analytics: leveraging mathematics to transform raw data into actionable information.
Sensitivity, Selectivity, and Reproducibility
One of the major achievements of this nanosensing platform is its limit of detection (LOD). The researchers reported nanomolar sensitivity, meaning that even trace concentrations of captopril, atenolol, or sildenafil could be reliably detected. This is especially important in pharmacokinetics, where drug levels fluctuate and require precise monitoring to avoid therapeutic failure or toxicity.
Equally important is selectivity. The nanosensor demonstrated minimal cross-reactivity with common biological interferents, ensuring that false positives were avoided. This is a vital quality, as serum, plasma, and urine are chemical jungles teeming with proteins, metabolites, and ions.
Finally, reproducibility was confirmed across multiple experimental runs, validating the robustness of the nanosensor. In the world of diagnostics, consistency is often more valuable than sensitivity, for without reproducibility, results cannot be trusted.
Implications for Clinical Pharmacology
The ability to simultaneously detect multiple drugs using a single, inexpensive nanosensor has far-reaching implications. In clinical pharmacology, this could mean:
- Therapeutic drug monitoring (TDM): Real-time detection of drug levels to tailor individual dosing.
- Adherence assessment: Distinguishing patients who are non-compliant from those who metabolize drugs differently.
- Polypharmacy safety: Monitoring drug interactions and avoiding toxic accumulation.
In resource-limited settings, such a nanosensor could revolutionize practice by replacing costly, infrastructure-heavy methods like LC-MS/MS with portable, low-cost alternatives.
Limitations and Challenges
Of course, no technology is without limitations. While the nanosensor shows great promise, its translation to clinical practice will face hurdles.
Firstly, biological matrices such as whole blood are more complex than the controlled environments used in the study. Proteins, lipids, and cellular debris could interfere with nanoparticle interactions. Secondly, regulatory pathways for diagnostic devices are rigorous. Demonstrating long-term stability, batch-to-batch reproducibility, and clinical validation will be necessary before adoption. Finally, scaling production of such sensors at low cost remains a technical challenge.
Yet, these challenges are not insurmountable. With advances in nanofabrication and portable spectroscopy, it is not far-fetched to imagine bedside nanosensors guiding drug therapy in real-time.
The Future of Nanosensing in Medicine
The implications of this work extend beyond captopril, atenolol, and sildenafil. The principle—using functionalized nanoparticles with multivariate analysis—can be applied to virtually any drug class. From antibiotics to anticancer agents, nanosensors could provide clinicians with immediate pharmacological insights.
Furthermore, integration with wearable technologies could allow continuous monitoring of drug levels through sweat, saliva, or interstitial fluid. The era of personalized pharmacotherapy, where drugs are not only prescribed but constantly monitored, may soon be upon us.
The irony, of course, is that while humans once marveled at the ability to swallow a pill and expect a generalized outcome, the future might involve tiny metallic particles ensuring that very pill is doing exactly what it is supposed to do.
Conclusion
The study on Gemifloxacin/Bergenin coated silver nanoparticles highlights the enormous potential of nanosensing in drug detection. By uniting nanotechnology, pharmacology, and statistical modeling, the researchers demonstrated a system capable of detecting three therapeutically significant drugs with high sensitivity, selectivity, and reproducibility.
This technology not only promises more efficient drug monitoring but also symbolizes the broader shift toward precision medicine. While challenges remain, the trajectory is clear: the next frontier of pharmacological monitoring will be miniature, metallic, and mathematically sophisticated.
FAQ
1. Why are nanoparticles better than traditional methods for drug detection?
Nanoparticles, especially silver, exhibit strong optical properties that are highly sensitive to surface interactions. When functionalized with suitable molecules, they can detect drugs at very low concentrations quickly and inexpensively, unlike chromatographic or mass spectrometric methods which are costlier and more labor-intensive.
2. Can this nanosensor be directly used in hospitals today?
Not yet. While the study shows excellent laboratory results, real-world biological fluids are more complex. Clinical validation, regulatory approval, and manufacturing standardization are necessary before such sensors can enter routine hospital use.
3. Could nanosensors be used for other drugs beyond the three tested here?
Absolutely. The principle is generalizable. By selecting appropriate surface ligands for nanoparticle functionalization, nanosensors could be tailored to detect antibiotics, anticancer drugs, hormones, or even illicit substances with equal efficiency.


