Introduction: The Lungs Under Siege
The lungs, though resilient in design, remain perilously vulnerable to chemical and inflammatory assaults. Among these, acute lung injury (ALI) stands as one of the most devastating, characterized by an overwhelming inflammatory response and the collapse of the alveolar-capillary barrier. Whether caused by sepsis, trauma, or chemical aspiration, ALI often escalates into acute respiratory distress syndrome (ARDS)—a clinical nightmare of hypoxemia and respiratory failure.
Hydrochloric acid (HCl) aspiration represents one of the most destructive culprits. In clinical contexts—such as anesthesia, stroke, or severe reflux—acid aspiration may rapidly trigger necrosis, hemorrhage, and massive inflammatory infiltration within the pulmonary tissue. Despite decades of research, the therapeutic landscape remains barren. Conventional pharmacological interventions—anti-inflammatory agents, antibiotics, and vasodilators—offer limited reprieve, often targeting symptoms rather than the biological root.
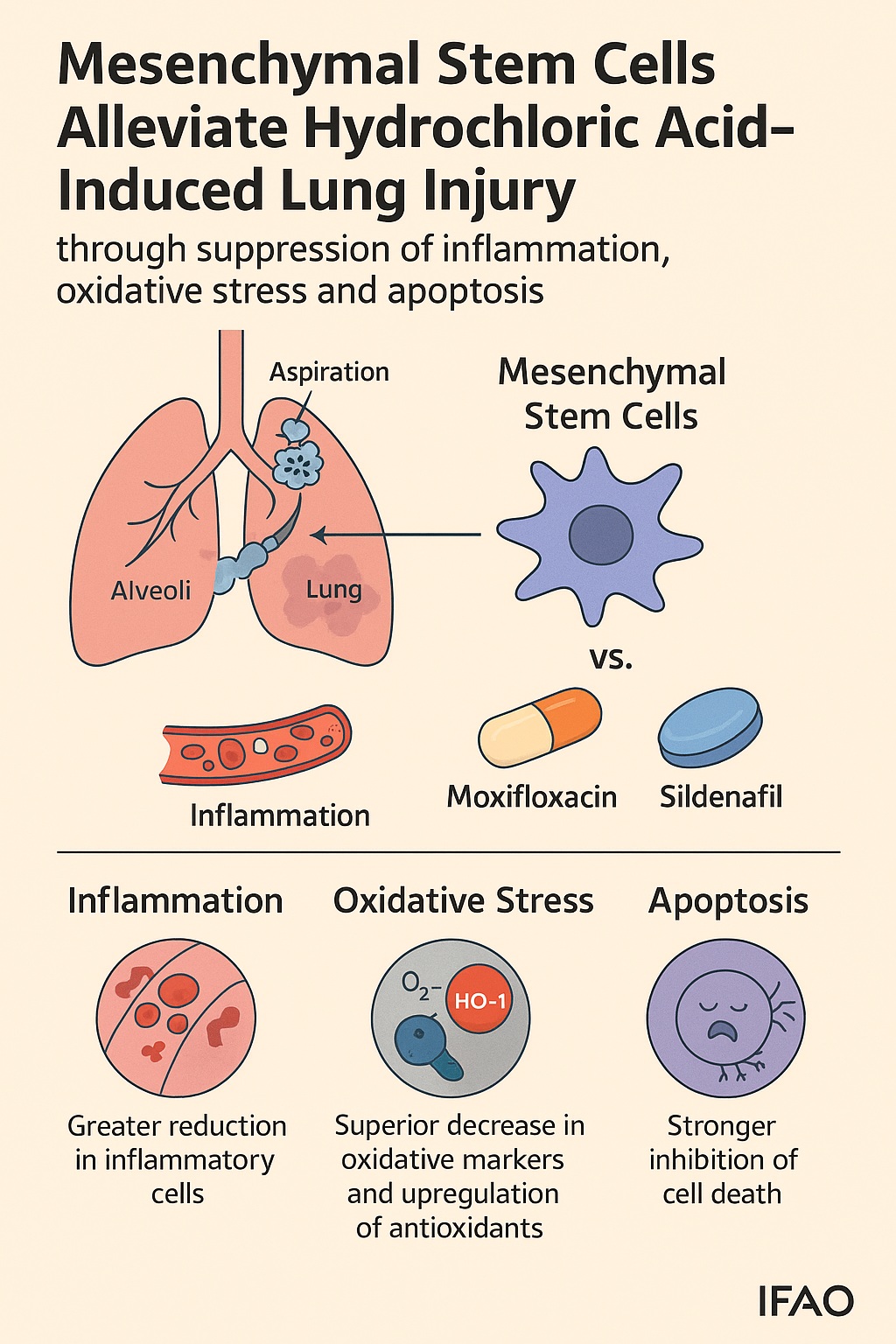
In this context, mesenchymal stem cells (MSCs) emerge as a biological revolution rather than a pharmacological compromise. These multipotent stromal cells, capable of immunomodulation, anti-oxidation, and tissue repair, offer a holistic therapeutic frontier. A recent experimental study by El-Metwaly and colleagues (2019) compared MSCs with two well-established agents—moxifloxacin, an antibiotic with anti-inflammatory properties, and sildenafil, a phosphodiesterase-5 inhibitor known for vasodilation and oxidative stress reduction. The results were striking: MSCs outperformed both pharmacological agents by mitigating inflammation, oxidative damage, and cellular apoptosis, suggesting a profound therapeutic promise for chemical-induced ALI.
Understanding Acute Lung Injury: When the Defense Becomes the Threat
The Pathophysiology of Self-Destruction
ALI is not simply an injury—it is a betrayal by the body’s own defenses. When the pulmonary epithelium encounters an insult such as aspirated HCl, it triggers a storm of cytokines, leading to massive recruitment of neutrophils, macrophages, and lymphocytes. These cells, while attempting to neutralize the invader, release reactive oxygen species (ROS) and proteolytic enzymes, which further damage the fragile alveolar walls. The result is a catastrophic breakdown of the alveolar–capillary interface, leading to pulmonary edema and impaired gas exchange.
The inflammatory cascade amplifies itself through molecules such as tumor necrosis factor-alpha (TNF-α), interleukin-6, and interleukin-8, creating a self-perpetuating cycle. Simultaneously, the production of antioxidant enzymes—superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH)—plummets, allowing oxidative stress to dominate. The cellular machinery succumbs to apoptosis, particularly through the activation of caspase-3, a key executioner enzyme in programmed cell death.
Thus, the pathology of ALI is not a matter of insufficient immune activity but excessive and misdirected zeal—the immune system’s equivalent of friendly fire.
The Role of Hydrochloric Acid in Chemical-Induced Injury
Aspiration of gastric contents introduces multiple damaging components—bacteria, enzymes, and bile—but the acidic component is the most destructive. Hydrochloric acid disrupts the lipid bilayer of pulmonary cells, coagulates proteins, and causes hemorrhagic necrosis. This not only injures alveolar epithelial cells but also increases vascular permeability, allowing fluid leakage and fibrin deposition. Over time, fibrosis develops, stiffening the lungs and locking the patient in a cycle of hypoxia and inflammation.
In animal models, such as those used by El-Metwaly et al., intratracheal administration of HCl reproducibly mimics the pathology of aspiration-induced ALI, offering a robust platform to test novel interventions.
Pharmacological Contenders: Moxifloxacin and Sildenafil
Moxifloxacin: Beyond Antimicrobial Action
Traditionally known as a fluoroquinolone antibiotic, moxifloxacin acts by inhibiting bacterial topoisomerase enzymes. However, emerging evidence suggests it also exerts immunomodulatory effects, attenuating neutrophil migration and cytokine production. These properties have sparked interest in its use beyond infection, particularly in inflammatory lung conditions.
In the El-Metwaly study, moxifloxacin-treated rats demonstrated modest improvements: reduced white blood cell counts, decreased lipid peroxidation (measured by malondialdehyde levels), and partial restoration of antioxidant enzymes. Yet, its effect plateaued—while it suppressed inflammation, it did not significantly alter apoptotic signaling or fully restore oxidative balance. It performed admirably as an antimicrobial agent dabbling in anti-inflammation, but it was, in essence, a tourist in the land of regeneration.
Sildenafil: The Vasodilator Turned Anti-Inflammatory Agent
Sildenafil, a phosphodiesterase-5 (PDE5) inhibitor, functions by preventing the degradation of cyclic guanosine monophosphate (cGMP). Elevated cGMP levels induce smooth muscle relaxation and vasodilation—principles well established in the treatment of erectile dysfunction and pulmonary hypertension.
Intriguingly, sildenafil also modulates inflammatory signaling and reduces oxidative stress. By enhancing cGMP-mediated pathways, it inhibits neutrophil activation and decreases superoxide formation. In ALI models, sildenafil improved oxygenation, decreased inflammatory cell infiltration, and mitigated tissue injury. However, much like moxifloxacin, it fell short of addressing the complex triad of inflammation, oxidation, and apoptosis that defines ALI. Its effects, though beneficial, were reactive rather than restorative.
The Stem Cell Revolution: Biology’s Built-In Repair System
The Nature of Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are the architects of repair in the body’s cellular hierarchy. Found in bone marrow, adipose tissue, umbilical cord, and other sources, these multipotent cells possess the remarkable ability to differentiate into osteoblasts, chondrocytes, and adipocytes. Yet, their greatest therapeutic contribution lies not in differentiation, but in their paracrine function—their ability to secrete growth factors, cytokines, and anti-inflammatory molecules that recalibrate damaged tissue environments.
In the context of lung injury, MSCs exhibit tropism toward inflamed regions, homing in on damaged alveoli. Once localized, they release hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), and interleukin-10, all of which suppress inflammation, stimulate angiogenesis, and inhibit apoptosis. Unlike synthetic drugs, MSCs engage the body’s intrinsic repair networks—a conversation between biology and biology, not chemistry and biology.
The Experiment: A Comparative Evaluation
In the study, rats with HCl-induced ALI were treated two hours post-injury with either moxifloxacin, sildenafil, a combination of both, or MSCs. After seven days, the results were clear: MSCs achieved superior outcomes across all parameters—hematologic, biochemical, genetic, and histological.
- Inflammation: MSCs markedly reduced total white blood cell and lymphocyte counts, reflecting potent immunosuppression.
- Oxidative stress: Antioxidant enzymes (SOD, CAT, GSH) were significantly restored, while malondialdehyde levels plummeted, indicating decreased lipid peroxidation.
- Apoptosis: The expression of caspase-3 was dramatically reduced in MSC-treated groups.
- Cellular defense: The expression of heme oxygenase-1 (HO-1)—a key enzyme in cellular protection—was strongly upregulated.
- Histology: Lung sections revealed near-normal architecture, with reduced hemorrhage, restored alveolar spaces, and diminished fibrosis.
In short, MSCs didn’t merely suppress injury—they orchestrated recovery.
The Triad of Protection: Inflammation, Oxidation, and Apoptosis
Calming the Inflammatory Storm
Inflammation is both the savior and the saboteur in lung injury. In the HCl model, inflammation rapidly escalates, attracting a flood of neutrophils and lymphocytes. Moxifloxacin and sildenafil tempered this response, but MSCs rewired it. Through their secretion of interleukin-1 receptor antagonists, transforming growth factor-beta (TGF-β), and other regulatory molecules, MSCs modulate both innate and adaptive immune responses.
They suppress pro-inflammatory cytokines while promoting the expression of anti-inflammatory macrophage phenotypes (M2). The result is not immunosuppression but immune recalibration—the difference between muting chaos and conducting a symphony.
Battling Oxidative Stress: Rebalancing the Redox Equation
Reactive oxygen species are the biochemical equivalent of friendly fire—useful in moderation, devastating in excess. In ALI, uncontrolled ROS generation leads to lipid peroxidation, protein oxidation, and DNA damage. MSCs combat this imbalance on two fronts: they restore enzymatic antioxidants such as SOD and CAT, and they stimulate the HO-1/Nrf2 pathway, the body’s intrinsic antioxidant defense network.
By contrast, moxifloxacin and sildenafil only partially improved oxidative balance. MSCs, through paracrine signaling, appear to reset redox homeostasis—a feat pharmacology has long aspired to but seldom achieved.
Blocking Apoptosis: Saving the Alveoli
Caspase-3 acts as the final executioner in the apoptotic pathway, cleaving cellular proteins and dismantling structural integrity. Elevated caspase-3 expression in lung tissue signifies extensive epithelial death. MSCs, by releasing hepatocyte growth factor and activating the anti-apoptotic Bcl-2 pathway, effectively reduce caspase-3 activity. This translates into preserved cellular viability and accelerated tissue repair.
While sildenafil and moxifloxacin reduced caspase activity to some extent, only MSCs achieved a biologically significant restoration of cell survival—a clear testament to their regenerative potency.
Histopathological Insights: Microscopic Evidence of Healing
Microscopic analysis paints a vivid picture of recovery. In untreated HCl-injured lungs, alveolar walls thicken, capillaries rupture, and hemorrhage fills the interstitial space—a landscape of destruction. Moxifloxacin and sildenafil reduced inflammation and hemorrhage modestly, but fibrosis and interstitial thickening persisted.
Conversely, MSC-treated lungs resembled near-normal tissue: expanded alveolar spaces, minimal inflammatory infiltration, and restored epithelial lining. The architectural repair suggests that MSCs not only halt progression but initiate structural regeneration, aligning with their documented ability to integrate into damaged parenchyma and secrete matrix remodeling enzymes.
Mechanistic Insights: How Stem Cells Orchestrate Recovery
Paracrine Signaling and Immunomodulation
MSCs act primarily through paracrine signaling rather than direct differentiation. They release bioactive molecules that modulate neighboring immune and structural cells. This molecular diplomacy leads to reduced infiltration of neutrophils and macrophages, balanced cytokine production, and enhanced angiogenesis.
HO-1 Pathway Activation
The upregulation of heme oxygenase-1 (HO-1) emerges as a central mechanism. HO-1 degrades pro-oxidant heme into biliverdin, carbon monoxide, and free iron—metabolites with potent anti-inflammatory and cytoprotective effects. By restoring HO-1 expression, MSCs effectively turn on the lungs’ built-in defense system against oxidative stress.
Anti-Apoptotic Crosstalk
Through secretion of growth factors like HGF, MSCs activate PI3K/Akt signaling, which suppresses caspase activity and stabilizes mitochondrial integrity. This pathway prevents apoptosis and promotes survival of alveolar epithelial cells, a critical determinant in reversing ALI pathology.
The Comparative Verdict: Cells Triumph Over Molecules
While both moxifloxacin and sildenafil provided measurable benefits, neither achieved comprehensive protection. Their effects were pharmacological patches on a biological rupture. MSCs, on the other hand, addressed the full triad of ALI pathology—inflammation, oxidative stress, and apoptosis—through dynamic and adaptive biological responses.
Importantly, MSC therapy operates in harmony with natural repair processes rather than overriding them. It is homeostatic medicine, restoring equilibrium rather than imposing chemical correction. This paradigm shift represents the future of regenerative pulmonary therapy.
Future Directions: From Bench to Bedside
Translating MSC therapy into clinical reality requires rigorous testing, standardization, and ethical oversight. Key challenges include:
- Ensuring consistent cell quality and viability across donors and batches.
- Preventing immune rejection and ensuring safe homing to target tissues.
- Defining optimal dosing and delivery routes—whether intravenous, intratracheal, or intraperitoneal.
Despite these hurdles, early-phase clinical trials in ARDS have already shown promising safety and feasibility profiles. Future strategies may combine MSCs with pharmacological agents such as sildenafil to synergistically enhance oxygenation and tissue perfusion.
The horizon of pulmonary medicine is shifting—from treating inflammation to reprogramming recovery.
Conclusion: Breathing New Life into Lung Therapy
The study by El-Metwaly and colleagues underscores a fundamental truth: the lungs do not merely need drugs—they need healing intelligence. Moxifloxacin and sildenafil can modulate; MSCs can regenerate. By suppressing inflammation, neutralizing oxidative stress, and inhibiting apoptosis, MSCs emerge not as experimental curiosities but as serious contenders in the fight against acute lung injury.
In an era where the respiratory system faces new global threats—from pollution to pandemics—therapies that restore rather than replace are the next frontier. MSC-based treatments promise to turn lung injury from a sentence into a story of recovery.
FAQ: Mesenchymal Stem Cells and Acute Lung Injury
1. Are mesenchymal stem cells safe for treating lung injury in humans?
Preclinical and early clinical data indicate that MSCs are generally safe and well-tolerated. Adverse immune reactions are rare, as these cells exhibit low immunogenicity. However, large-scale trials are ongoing to confirm long-term safety and efficacy.
2. Can MSC therapy replace conventional drugs like moxifloxacin or sildenafil?
Not yet. While MSCs show superior regenerative capacity, antibiotics and vasodilators remain essential in specific contexts, especially where infection or hemodynamic instability coexists. The future likely lies in combination therapy, leveraging both biological and pharmacological strengths.
3. How soon could MSC therapy become standard for acute lung injury?
Clinical translation is progressing rapidly. With successful ongoing trials in ARDS and other inflammatory lung diseases, therapeutic MSC use may become clinically mainstream within the next decade, pending regulatory approval and manufacturing standardization.