Introduction
In an age where modern pharmacology often looks toward synthetic innovation, it is somewhat ironic that some of the most potent modulators of sexual health are found not in laboratories but in nature’s pharmacopoeia. Among these, three names have long echoed in both ancient Ayurvedic scrolls and contemporary biomedical literature: Mucuna pruriens, Withania somnifera (Ashwagandha), and Tribulus terrestris. Each of these botanicals has, in its own right, been hailed as a natural aphrodisiac, endocrine modulator, and adaptogen. Yet, until recently, their synergistic potential remained largely theoretical.
The study titled “MAT, a Novel Polyherbal Aphrodisiac Formulation, Enhances Sexual Function and Nrf2/HO-1 Pathway While Reducing Oxidative Damage in Male Rats” sought to change that. By combining these three herbs in defined proportions, researchers designed MAT, a polyherbal formulation tested against both a negative control and a sildenafil citrate (Viagra®) benchmark in male rats. What emerged was an intricate story of hormonal restoration, oxidative balance, and molecular orchestration — one that connects traditional herbal wisdom with the precision of molecular biology.
At its core, the MAT study challenges a long-held dichotomy: that natural remedies and mechanistic pharmacology are mutually exclusive. On the contrary, it elegantly demonstrates that polyherbal formulations can act on defined biochemical pathways, namely the Nrf2/HO-1 antioxidant axis and NF-κB inflammatory signaling, with precision comparable to — and, in some respects, surpassing — synthetic agents.
The Burden of Male Sexual Dysfunction: A Modern Pathophysiological Puzzle
Sexual dysfunction in men, encompassing erectile failure, reduced libido, and impaired spermatogenesis, represents a growing clinical burden. Despite advances in urology and endocrinology, nearly half of all infertility cases involve a male factor, often rooted in subtle molecular imbalances rather than overt pathology. Beyond vascular or neurological origins, oxidative stress has emerged as a central player — a biochemical saboteur that compromises sperm integrity, damages testicular tissue, and disrupts androgen synthesis.
Reactive oxygen species (ROS), though necessary in small amounts for sperm capacitation and acrosomal reactions, can, in excess, wreak havoc on sperm DNA, lipids, and proteins. When endogenous antioxidant defenses falter, lipid peroxidation products like malondialdehyde (MDA) accumulate, signaling oxidative distress and reduced fertility potential. This oxidative imbalance is often exacerbated by lifestyle factors such as psychological stress, pollution, metabolic disease, and even pharmacologic treatments themselves.
Here lies the rationale for integrating antioxidant-rich botanicals into male fertility management. Unlike single-molecule antioxidants, polyherbal formulations such as MAT offer multi-targeted redox modulation, combining direct scavenging effects with upregulation of endogenous antioxidant defenses. This dual action — both suppressing ROS and enhancing cellular resilience — is what distinguishes MAT’s mechanistic footprint from conventional treatments.
MAT’s Composition: An Intelligent Botanical Triad
The MAT formulation brings together Mucuna pruriens, Ashwagandha, and Tribulus terrestris — three plants with well-documented effects on sexual performance, fertility, and oxidative stress.
Mucuna pruriens (velvet bean) is rich in L-DOPA, a dopamine precursor that influences libido and sexual motivation via central neuroendocrine pathways. It also exhibits antioxidant properties that protect testicular tissue from oxidative injury.
Ashwagandha (Withania somnifera), revered as a Rasayana in Ayurvedic medicine, functions as an adaptogen, attenuating stress-induced cortisol elevation while promoting testosterone synthesis and sperm quality.
Tribulus terrestris, a cornerstone of traditional aphrodisiac therapy, contains protodioscin — a steroidal saponin believed to enhance androgen receptor density and stimulate luteinizing hormone (LH) release.
In the MAT study, these herbs were combined in two distinct doses:
- MAT1: 10 mg/kg each of Mucuna, Ashwagandha, and Tribulus
- MAT2: 20 mg/kg each
Both formulations were administered for eight weeks to male Sprague-Dawley rats. For benchmarking, a group received sildenafil citrate (5 mg/kg), allowing direct comparison between a synthetic phosphodiesterase inhibitor and the natural formulation.
The underlying question was elegant in its simplicity: Can nature, through biochemical synergy, rival pharmacology’s most iconic molecule in restoring sexual vitality?
Hormonal Symphony: Testosterone, FSH, and LH Elevation
MAT’s effects on endocrine parameters were profound. Both MAT1 and MAT2 significantly elevated serum testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) compared to controls. Notably, the increases were dose-dependent, with MAT2 outperforming MAT1 and closely paralleling sildenafil-treated animals in androgenic response.
Mechanistically, this enhancement reflects stimulation at multiple endocrine tiers. LH acts on Leydig cells to promote testosterone synthesis, while FSH supports Sertoli cell function and spermatogenesis. By enhancing both, MAT effectively restores the hypothalamic–pituitary–gonadal axis, the hormonal triad governing male fertility.
Interestingly, testosterone levels in MAT2 rats were approximately 1.7 times higher than controls, suggesting a physiologically relevant upregulation rather than an artificial pharmacologic surge. Such balanced elevation carries less risk of feedback suppression or metabolic desensitization — a potential advantage over exogenous androgenic drugs.
From a translational standpoint, these findings highlight the endocrine harmonization potential of polyherbal therapy: rather than forcing hormonal changes, MAT appears to re-tune the orchestra of male reproductive hormones through natural modulation.
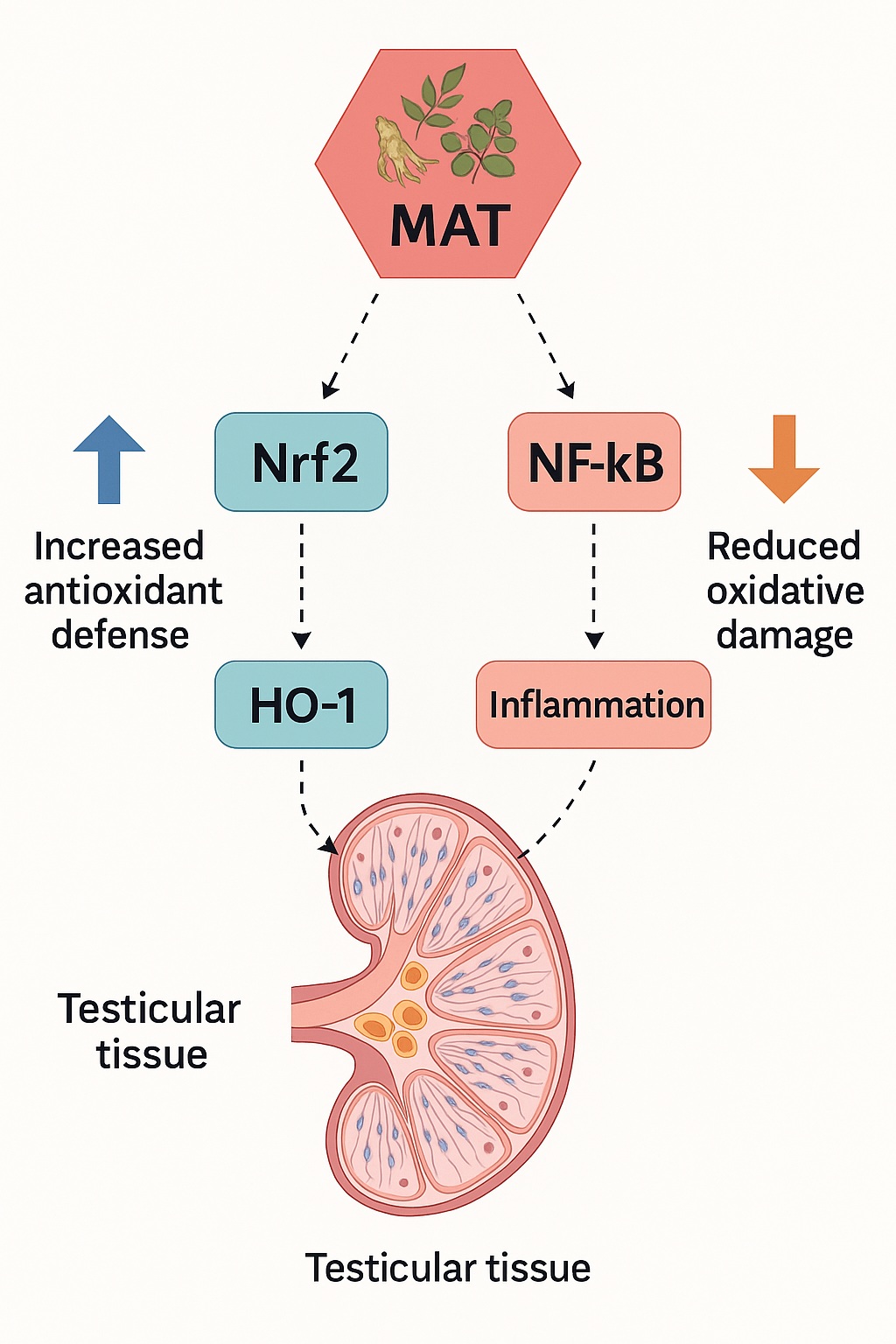
The Molecular Ballet: Nrf2/HO-1 Activation and NF-κB Inhibition
Beneath the surface of hormonal shifts lies a molecular choreography central to MAT’s therapeutic profile: the activation of the Nrf2/HO-1 antioxidant defense pathway and suppression of the NF-κB inflammatory cascade.
Nrf2 (nuclear factor erythroid 2–related factor 2) is a master transcriptional regulator of cellular antioxidant defense. Upon activation, it binds to antioxidant response elements (AREs) in DNA, triggering the expression of detoxifying enzymes such as heme oxygenase-1 (HO-1). Together, these proteins neutralize reactive intermediates, limit lipid peroxidation, and stabilize redox homeostasis.
Conversely, NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) governs the transcription of pro-inflammatory cytokines and apoptosis regulators. Under oxidative conditions, NF-κB activation exacerbates tissue injury and compromises sperm viability.
MAT administration, particularly at higher doses, upregulated Nrf2 and HO-1 while downregulating NF-κB, effectively restoring molecular balance. This bidirectional modulation not only reduced oxidative load but also mitigated inflammation-driven testicular stress.
In essence, MAT acts not as a blunt antioxidant but as a molecular conductor, orchestrating the cellular response to oxidative challenge by fine-tuning both protective and inflammatory signaling networks.
Oxidative Stress and MDA Reduction: Quantifying Protection
Among the study’s most quantifiable outcomes was the significant reduction in malondialdehyde (MDA) — a marker of lipid peroxidation and oxidative stress. Both serum and testicular MDA levels were markedly lower in MAT-treated groups, especially MAT2, which achieved over 30% reduction relative to controls.
This biochemical shift corroborates the upregulation of Nrf2/HO-1 observed in western blot analyses. By reducing MDA accumulation, MAT effectively shielded sperm membranes from peroxidative damage, preserving motility and viability. The result is not merely antioxidant protection but functional restoration — a distinction crucial in reproductive biology, where structure and function are inseparable.
Interestingly, sildenafil, despite its vascular benefits, did not reduce MDA to the same extent. This highlights a fundamental divergence in mechanism: where sildenafil enhances hemodynamics, MAT addresses the metabolic and oxidative substrate of dysfunction, targeting the root rather than the symptom.
Fertility Metrics: Sperm Count, Motility, and Morphology
Reproductive success ultimately depends on sperm performance — a domain where MAT excelled. Both formulations significantly increased sperm count and motility, with MAT2 showing the highest efficacy. The increase in sperm motility exceeded 30%, and sperm concentration rose by up to 1.5-fold compared to controls. Morphological integrity was also maintained, with minimal abnormalities observed.
These outcomes reflect the synergistic interplay between enhanced androgenic drive, improved redox balance, and cellular protection. Mucuna’s dopaminergic influence likely enhanced libido and ejaculatory reflexes, while Tribulus and Ashwagandha supported spermatogenesis through endocrine and antioxidant mechanisms.
From a physiological perspective, the MAT results reinforce a core principle: sperm quality is as much a reflection of systemic oxidative equilibrium as it is of testicular function. By reducing oxidative burden and supporting hormonal output, the formulation creates a biochemical environment conducive to optimal sperm maturation and motility.
Safety and Systemic Tolerance: The Absence of Collateral Damage
Efficacy without safety is a pyrrhic victory. Encouragingly, MAT administration did not alter hepatic or renal function markers — including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), or creatinine. This biochemical neutrality indicates systemic safety and metabolic compatibility over the eight-week exposure.
The absence of organ toxicity differentiates MAT from many synthetic aphrodisiacs, which, while potent, can impose hepatic or cardiovascular strain. Polyherbal formulations, by virtue of their complex phytochemical matrices, often distribute metabolic burden across multiple pathways, reducing single-organ toxicity.
Clinically, this positions MAT as a potentially sustainable adjunct for long-term use, especially in chronic sexual dysfunction or subfertility cases where continuous pharmacotherapy would otherwise be contraindicated.
Seminal Vesicles and Prostate: Androgenic Echoes
An intriguing observation from the study was the increase in seminal vesicle and ventral prostate weights in MAT-treated animals, paralleling the sildenafil group. Far from indicating pathology, these hypertrophic changes reflect androgenic stimulation and increased secretory activity of accessory glands — essential components of semen production and fertility.
Androgens drive both the growth and secretory function of these organs. Therefore, the increased glandular weight observed with MAT treatment aligns with the hormonal surge in testosterone and LH. However, these changes remained within physiological bounds, indicating functional enhancement rather than hyperplasia.
This reinforces a broader theme: MAT does not disrupt endocrine homeostasis but rather restores it toward a more youthful equilibrium, enhancing anabolic reproductive parameters without pathological overstimulation.
Mechanistic Integration: The Network Pharmacology Perspective
From a systems biology standpoint, MAT’s success is best explained through network pharmacology — the concept that multi-component herbal formulations exert therapeutic effects through simultaneous modulation of multiple biological nodes. Each component contributes distinct but overlapping effects on redox balance, endocrine function, and cellular signaling.
- Mucuna pruriens: dopaminergic and antioxidative, enhancing libido and spermatogenesis.
- Ashwagandha: adaptogenic and endocrine supportive, reducing cortisol and promoting testosterone synthesis.
- Tribulus terrestris: steroidogenic and vasodilatory, stimulating LH and improving penile hemodynamics.
The convergence of these actions produces an outcome greater than the sum of its parts. This phytochemical synergy aligns with emerging pharmacological models that favor multi-target modulation over single-receptor interventions, particularly for complex physiological systems like sexual function.
Redefining Natural Aphrodisiacs: From Folklore to Mechanistic Science
Historically, herbal aphrodisiacs occupied a nebulous space between myth and medicine. They were celebrated by traditional healers yet dismissed by clinical scientists for lack of mechanistic clarity. The MAT study bridges that gap, translating traditional herbal synergy into a molecularly validated pharmacological framework.
By demonstrating quantifiable effects on the Nrf2/HO-1 and NF-κB pathways, the research elevates these botanicals from anecdotal aphrodisiacs to legitimate redox modulators with systemic implications. This shift in perception is more than academic — it heralds a reconciliation between ethnomedicine and molecular biology, validating centuries of empirical wisdom through the lens of modern science.
Indeed, the results suggest that sexual health and oxidative resilience are biochemically intertwined, and that treating one necessarily influences the other. The MAT formulation, in this respect, represents a prototype for rationally designed phytopharmacology — where traditional herbs are combined not by folklore but by molecular logic.
Conclusion
The MAT polyherbal formulation — integrating Mucuna pruriens, Ashwagandha, and Tribulus terrestris — represents a significant stride in the evolution of natural therapeutics for male sexual dysfunction. By enhancing androgenic hormones, improving sperm quality, and modulating redox and inflammatory pathways, MAT achieves a holistic restoration of reproductive vitality.
Its mechanisms — upregulation of Nrf2/HO-1, inhibition of NF-κB, and reduction of MDA — collectively protect the testicular microenvironment from oxidative injury, facilitating hormonal recovery and spermatogenic efficiency. Unlike monomolecular drugs, MAT’s polypharmacology allows a gentle, adaptive correction of dysfunction, making it a promising template for integrative therapeutics.
In a medical world increasingly dominated by synthetic interventions, MAT reminds us that nature’s complexity is not a flaw to be simplified, but a strategy to be understood. The future of sexual health may well lie at the intersection of ancient botany and modern biochemistry.
FAQ: MAT and Male Sexual Health
1. What makes MAT different from single-herb supplements?
MAT combines three botanicals that act on complementary pathways — hormonal, antioxidant, and anti-inflammatory — creating synergistic efficacy greater than any single extract alone.
2. How does MAT enhance fertility on a molecular level?
It activates the Nrf2/HO-1 antioxidant pathway, reduces NF-κB–mediated inflammation, and restores hormonal balance, thereby protecting sperm from oxidative damage and improving motility.
3. Can MAT be considered a natural alternative to sildenafil?
While sildenafil primarily improves penile blood flow, MAT targets the underlying oxidative and hormonal mechanisms of sexual dysfunction. It offers a slower but more systemic restoration, potentially suitable for long-term support.