Introduction
Erectile dysfunction (ED) remains one of the most distressing sequelae following radical prostatectomy. While surgeons have refined nerve-sparing techniques since the 1980s, the sobering truth persists: many men continue to experience compromised erectile function after surgery. Beyond its physical implications, postprostatectomy ED exerts a disproportionate psychological burden on patients and their partners, often overshadowing oncological triumphs.
Penile rehabilitation has therefore become a central topic in urological practice. The armamentarium includes phosphodiesterase type 5 inhibitors (PDE5Is), intracavernosal injections, intraurethral or topical prostaglandins, and vacuum erection devices. Yet, despite decades of innovation, an “ideal” protocol has remained elusive. Into this therapeutic gap steps low-intensity extracorporeal shockwave therapy (LI-ESWT), a noninvasive modality that has garnered increasing attention.
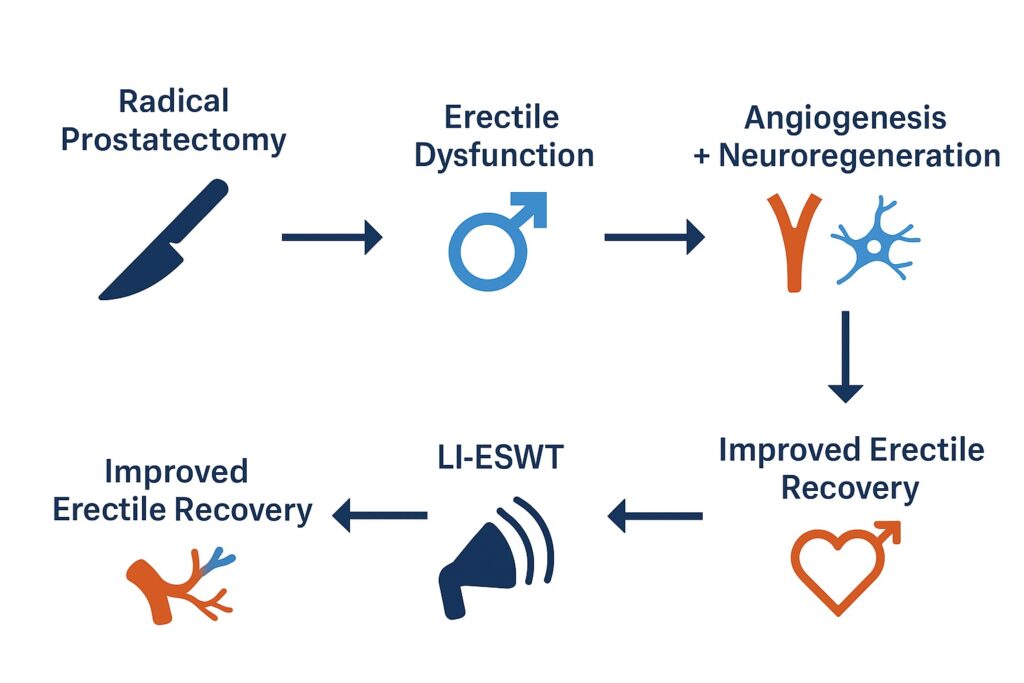
Originally explored for vasculogenic ED, LI-ESWT is now being investigated as a regenerative tool for men recovering from prostate surgery. The therapy’s promise lies in its ability to induce angiogenesis, stimulate Schwann cell proliferation, and promote neuronal nitric oxide synthase activity—mechanisms potentially capable of reversing neuropraxia and vascular compromise inherent to radical prostatectomy.
This article critically reviews the current evidence, explains the biological rationale, and offers a pragmatic reflection on the future role of LI-ESWT in penile rehabilitation after prostate cancer surgery.
The Biological Basis of Shockwave Therapy
Shockwave therapy, in its low-intensity form, applies acoustic waves to deep tissue, producing controlled microtrauma and shear stress. The paradoxical result of this “injury” is regeneration rather than damage. By activating cellular repair mechanisms, LI-ESWT triggers cascades of biochemical signals that enhance blood vessel formation and neuronal recovery.
Preclinical research has shown that LI-ESWT upregulates vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS). Together, these promote neovascularization, improve penile hemodynamics, and preserve smooth muscle integrity. Additionally, experiments in rodent models with cavernous nerve injury demonstrated that LI-ESWT increases Schwann cell proliferation and neuronal nitric oxide synthase activity, suggesting potential neurotrophic effects.
In essence, LI-ESWT is not merely symptomatic therapy but a form of regenerative urology, aiming to restore structural and functional integrity to penile tissue damaged by surgery. This conceptual leap differentiates it from pharmacological agents that simply enhance what remains of erectile physiology.
Challenges of Postprostatectomy Erectile Dysfunction
The pathophysiology of ED after radical prostatectomy is complex. Even when nerves are spared, patients often suffer from neuropraxia due to traction, compression, coagulation, ischemia, or inflammatory damage to the neurovascular bundle. Arterial injury, particularly to accessory pudendal arteries, further compounds erectile impairment.
Recovery is hampered by penile hypoxia, smooth muscle apoptosis, and fibrosis, which evolve during the postoperative period. Without timely intervention, these changes become irreversible. Hence the concept of penile rehabilitation—early, structured interventions designed not only to achieve erections but to preserve erectile tissue for long-term function.
It is precisely within this window that LI-ESWT could exert its greatest value, enhancing perfusion and neuronal recovery before chronic fibrosis sets in.
Evidence from Clinical Studies
To date, nine clinical studies—published between 2015 and 2022—have explored LI-ESWT in men with postprostatectomy ED. Their characteristics reveal both enthusiasm and heterogeneity.
- Designs and scale: Only three were randomized controlled trials (RCTs). Others were small nonrandomized comparative or single-center studies, with total patient numbers scarcely exceeding 230 across all reports.
- Treatment protocols: Protocols varied dramatically, from five to twelve sessions, one to three per week, with energy flux densities ranging from 0.09 to 0.30 mJ/mm². Some studies started treatment within days after surgery, others six months later.
- Adjunctive therapies: Most studies compared LI-ESWT plus daily PDE5Is (often tadalafil 5 mg) versus PDE5Is alone. Only one trial examined LI-ESWT against a sham protocol.
- Outcomes: Improvements were often reported in International Index of Erectile Function (IIEF-5) scores and Erection Hardness Score (EHS). Yet, not all improvements reached statistical or clinical significance, reflecting small sample sizes and variable follow-up.
One consistent theme emerges: combination therapy of LI-ESWT plus PDE5Is tends to outperform PDE5Is alone, albeit modestly. The question remains whether this reflects true biological regeneration or simply short-term symptomatic benefit.
Timing: Early Versus Delayed Intervention
An intriguing aspect of the literature is the question of timing. Should LI-ESWT be initiated immediately after catheter removal, or is it more effective months later?
Some authors advocate for early initiation, reasoning that enhanced cavernosal perfusion may prevent hypoxia-induced remodeling. Indeed, early LI-ESWT showed better sexual function recovery in one Japanese study compared with delayed treatment, though confounding factors such as differences in nerve-sparing rates limited definitive conclusions.
Others suggest that delayed therapy may target fibrosis reversal, a different but equally relevant mechanism. The truth may be less binary: LI-ESWT could serve dual roles, early as a preventive measure and later as a restorative intervention.
The Limitations of Current Evidence
The allure of LI-ESWT is undeniable, but sober assessment of the literature exposes significant weaknesses:
- Sample size: Most studies include fewer than 50 patients, undermining statistical power.
- Heterogeneity: Differences in protocols, shockwave devices, and outcome measures render cross-comparison problematic.
- Bias and design flaws: High risk of bias per GRADE assessment, limited blinding, and short follow-up intervals limit reliability.
- Endpoints: Not all statistically significant improvements translate into meaningful patient outcomes. Erectile hardness and IIEF scores are proxies; what patients truly value is return to baseline function and sexual satisfaction.
These limitations demand larger, multicenter, rigorously controlled trials before LI-ESWT can be recommended as standard of care.
Integration with Current Rehabilitation Protocols
Despite the gaps in evidence, clinicians must often make real-world decisions. How might LI-ESWT be integrated into penile rehabilitation today?
One pragmatic model involves multimodal therapy: PDE5Is daily to maintain cGMP activity, LI-ESWT sessions to promote tissue regeneration, and adjunctive use of vacuum devices or intracavernosal injections as needed. This layered approach acknowledges that no single therapy addresses all dimensions of postprostatectomy ED.
Another consideration is patient selection. Men undergoing bilateral nerve-sparing surgery are more likely to benefit from LI-ESWT than those with complete neurovascular bundle resection. Tailoring therapy based on surgical details and baseline erectile function remains critical.
Beyond Erectile Function: Other Outcomes
It is tempting to view LI-ESWT solely through the lens of erections. Yet, two studies explored its effect on urinary continence recovery, with no significant differences compared to controls. While disappointing, this aligns with the biological rationale: LI-ESWT primarily targets vascular and neuronal elements of penile tissue, not sphincteric mechanisms.
Nonetheless, broader quality-of-life endpoints—sexual bother, intimacy restoration, and psychological well-being—must also be integrated into future assessments. Erectile rigidity alone does not define recovery for patients and partners navigating post-cancer survivorship.
Conclusion
LI-ESWT represents one of the most intriguing frontiers in sexual medicine. By harnessing principles of tissue regeneration, it aspires to move penile rehabilitation beyond pharmacology into the realm of restorative therapy. The current evidence, while limited and heterogeneous, suggests potential benefit, especially when combined with PDE5 inhibitors.
However, enthusiasm must be tempered with caution. Larger RCTs, standardized protocols, and longer follow-up are essential before LI-ESWT can be considered more than an experimental adjunct. For now, it remains a promising but unproven ally in the fight against postprostatectomy erectile dysfunction.
The broader lesson is clear: true rehabilitation after prostate cancer surgery requires more than tumor eradication. It demands attention to the vascular, neuronal, and psychosocial dimensions of male health—dimensions LI-ESWT may one day help restore.
FAQ
1. What is LI-ESWT, and how does it work in erectile dysfunction?
Low-intensity extracorporeal shockwave therapy delivers acoustic energy to penile tissue, inducing controlled microtrauma that stimulates angiogenesis, neuronal recovery, and improved blood flow.
2. Is LI-ESWT currently recommended for men with postprostatectomy ED?
Not yet. Evidence from small trials suggests benefit, but larger randomized studies are required before it can be considered standard therapy. At present, it may be offered in research or highly selected clinical settings.
3. Does LI-ESWT replace PDE5 inhibitors after prostate surgery?
No. Most studies show the best outcomes when LI-ESWT is combined with PDE5 inhibitors. It is best viewed as a potential complement rather than a substitute.