Introduction
Acute kidney injury (AKI) remains one of the most feared complications following cardiac surgery. Despite improvements in surgical technique, cardiopulmonary bypass (CPB) protocols, and perioperative care, up to one-third of patients undergoing major cardiac operations develop postoperative AKI. This complication is far from trivial: it increases intensive care stay, drives multi-organ dysfunction, and quadruples postoperative mortality. Unfortunately, preventive strategies remain elusive, and no pharmacological therapy has been validated as reliably protective.
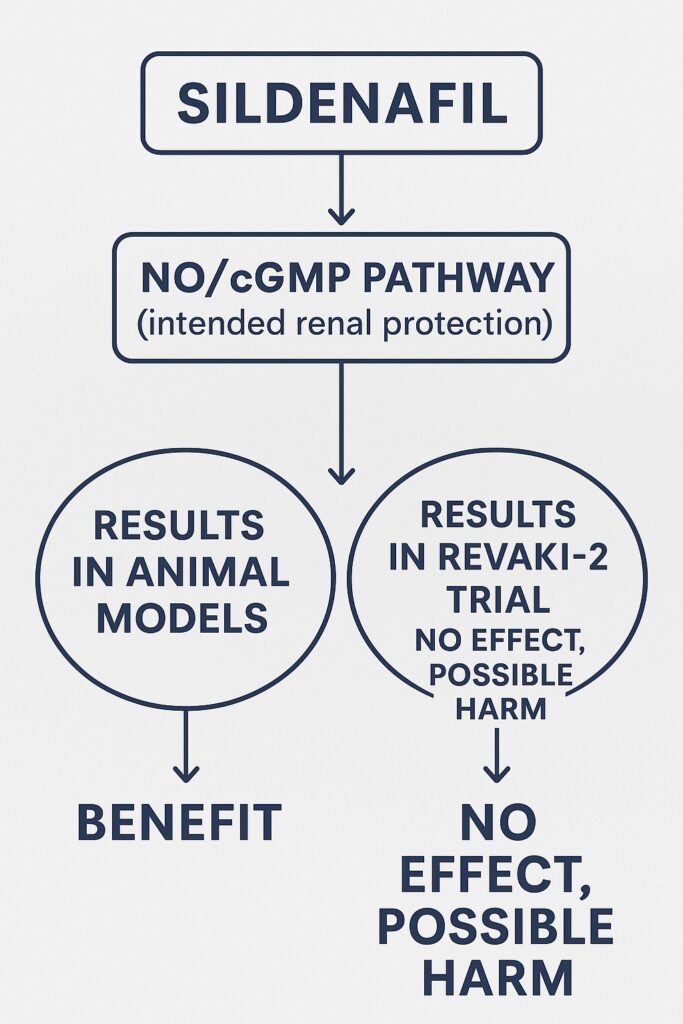
Against this background, sildenafil citrate—a drug better known for erectile dysfunction and pulmonary hypertension—entered the scene as a potential renal protector. Its mechanism, rooted in nitric oxide (NO) preservation and cyclic guanosine monophosphate (cGMP) signaling, seemed biologically attractive. Preclinical swine models demonstrated that intravenous sildenafil reduced post-CPB AKI, rekindling hopes for clinical translation. Thus, the REVAKI-2 trial was launched to test whether these findings could be replicated in humans at risk of postoperative kidney injury.
What followed was a sobering but scientifically invaluable lesson: promising animal data do not always survive the harsh scrutiny of randomized controlled trials (RCTs).
The Rationale: From Erectile Function to Renal Protection
Sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, works by preserving cGMP levels in smooth muscle, enhancing vasodilation, and maintaining NO bioactivity. While its fame stems from urological and cardiopulmonary applications, its potential to protect the kidney from ischemia-reperfusion injury derives from three theoretical advantages:
- Improved Renal Blood Flow – Sildenafil-mediated vasodilation could maintain microcirculatory perfusion during and after CPB.
- Endothelial Protection – By sustaining NO availability, sildenafil might blunt endothelial injury and oxidative stress.
- Anti-inflammatory Effects – Preclinical studies suggested reduced leukocyte activation and cytokine release under PDE5 inhibition.
These mechanisms supported the hypothesis that intravenous sildenafil could act as a pharmacological shield against CPB-induced renal damage.
The REVAKI-2 Trial: Design and Methods
The REVAKI-2 trial was a rigorously designed, double-blind, placebo-controlled RCT conducted at a single tertiary UK cardiac surgery center. Patients at elevated risk of AKI (median risk score ~30%) undergoing valve or combined valve–coronary artery bypass graft (CABG) surgery were randomized to receive either:
- Sildenafil citrate: 12.5 mg intravenously over 150 minutes starting at skin incision.
- Placebo: 5% dextrose administered in an identical manner.
A total of 129 patients were enrolled (60 sildenafil, 69 placebo). Participants were followed until hospital discharge and again at 6 weeks and 3 months postoperatively.
The primary endpoint was serum creatinine trajectory over 96 hours. Secondary outcomes included estimated glomerular filtration rate (eGFR), multiple organ dysfunction scores (MODS), biomarkers of inflammation and kidney injury, extubation and discharge times, and composite clinical events.
Patient Characteristics
The median age was 72 years, with nearly 80% of participants being male. Baseline renal function was moderately reduced (mean eGFR ~70–75 mL/min/1.73 m²). Diabetes was present in 40% of patients, reflecting a high comorbidity burden. Surgical procedures included valve replacement, CABG, and combined operations.
The sildenafil and placebo groups were well balanced, though more patients in the sildenafil arm underwent redo surgery and had slightly lower baseline eGFR. Importantly, these imbalances were addressed through sensitivity analyses, which confirmed robustness of findings.
The Primary Outcome: No Renal Protection
The central finding was stark: intravenous sildenafil did not reduce serum creatinine after cardiac surgery. Across all postoperative time points, creatinine trends were nearly identical between sildenafil and placebo groups. The adjusted mean difference was a negligible 0.88 µmol/L (95% CI: –5.82 to 7.59; P=0.797).
Equally, no subgroup—including those stratified by eGFR or surgical type—demonstrated benefit. Even after excluding redo surgeries in a post hoc sensitivity analysis, the absence of effect persisted.
This result directly contradicted the protective effects seen in preclinical models and underscored the translational gap between experimental and human physiology.
Secondary Outcomes: A Hint of Harm?
If lack of benefit was disappointing, the secondary outcomes added nuance.
- eGFR: No significant difference up to 6 weeks postoperatively.
- Biomarkers: Levels of NGAL, Timp2*IGFBP7, and inflammatory cytokines (IL-6, IL-8, IL-10) were unchanged between groups.
- Clinical events: Mortality, sepsis, myocardial injury, and composite outcomes did not differ significantly.
However, there was one surprising signal: MODS were higher in the sildenafil group (mean difference: 0.54; P=0.044). Though the absolute increase was small and likely not clinically meaningful, the finding raised eyebrows. Elevated bilirubin and troponin appeared to drive this difference, hinting at subtle liver and myocardial stress.
Thus, not only did sildenafil fail to protect, but it might even have tilted physiology toward fragility—though this interpretation remains speculative.
Strengths and Limitations of the Trial
REVAKI-2 was methodologically robust:
- Double-blind, randomized design with concealed allocation.
- Rigorous eligibility criteria enriched for high-risk patients.
- Careful biomarker sampling alongside clinical endpoints.
- Low attrition and strong protocol adherence.
Yet, limitations temper interpretation:
- Sample size: Although powered for creatinine differences, the study was not large enough to detect subtle but clinically relevant outcome shifts.
- Primary endpoint sensitivity: Serum creatinine, though practical, is a blunt instrument for AKI detection. More sensitive biomarkers or long-term renal outcomes might reveal nuances.
- Duration of therapy: A single intraoperative infusion may not have been long enough. Other agents, like atrial natriuretic peptide, show benefits only when sustained for several days.
Still, within its design, the trial offers a clear conclusion: intravenous sildenafil is not renoprotective in adult cardiac surgery.
Translational Lessons: Why Did Sildenafil Fail?
The discordance between animal and human results invites reflection. Several factors may explain the translational disappointment:
- Complex human physiology: Aging, diabetes, atherosclerosis, and polypharmacy create biological landscapes far removed from controlled swine models.
- Timing and dosing: In humans, perioperative NO dynamics and oxidative stress may require prolonged modulation rather than a short infusion.
- Alternative pathways: AKI in CPB is multifactorial—hemolysis, inflammation, ischemia, venous congestion—many of which PDE5 inhibition may not meaningfully affect.
In short, the pathophysiology of human AKI is too diverse for sildenafil’s relatively narrow mechanism to overcome.
Clinical Implications
The clinical message is straightforward but crucial: sildenafil should not be used for renal protection in cardiac surgery patients. It does not improve kidney outcomes, and may even carry subtle risks.
For practitioners, the emphasis should remain on proven strategies: meticulous perioperative hemodynamic management, avoidance of nephrotoxins, judicious transfusion, and timely renal replacement therapy when indicated.
This trial also reinforces the necessity of evidence-based translation: therapies must be validated in humans before widespread use, no matter how convincing animal models may appear.
Conclusion
The REVAKI-2 trial delivers a sobering but necessary message: despite elegant theoretical rationale and strong preclinical support, intravenous sildenafil does not prevent AKI in adult cardiac surgery. Its failure highlights the complexity of perioperative kidney injury and the challenges of translating laboratory success into clinical benefit.
In an era hungry for renoprotective strategies, sildenafil will not be the answer. But the trial provides a valuable foundation for future research, emphasizing the need for multi-targeted approaches that account for the multifactorial nature of CPB-induced AKI.
For now, sildenafil should remain in its established roles: treatment of erectile dysfunction and pulmonary hypertension, not as a renal savior in the operating theater.
FAQ
1. Why was sildenafil expected to protect the kidneys during surgery?
Because it enhances nitric oxide signaling, sildenafil was hypothesized to preserve renal blood flow, reduce oxidative stress, and limit ischemia-reperfusion injury.
2. Did the REVAKI-2 trial show any benefit at all?
No. The trial found no reduction in serum creatinine, eGFR decline, or AKI biomarkers. In fact, a small increase in multiple organ dysfunction scores was observed in the sildenafil group.
3. Should clinicians consider using sildenafil for kidney protection in cardiac surgery?
No. Based on the REVAKI-2 results, sildenafil should not be used for this purpose. Its role remains restricted to erectile dysfunction and pulmonary hypertension.