Introduction
The intrauterine environment represents one of the most intricate pharmacological frontiers in modern medicine. The capacity to administer therapeutics directly to the developing fetus—bypassing maternal metabolism and placental barriers—has long been considered a bold aspiration in fetal medicine. Recent advances in molecular pharmacology, imaging, and minimally invasive procedures have brought this vision closer to reality.
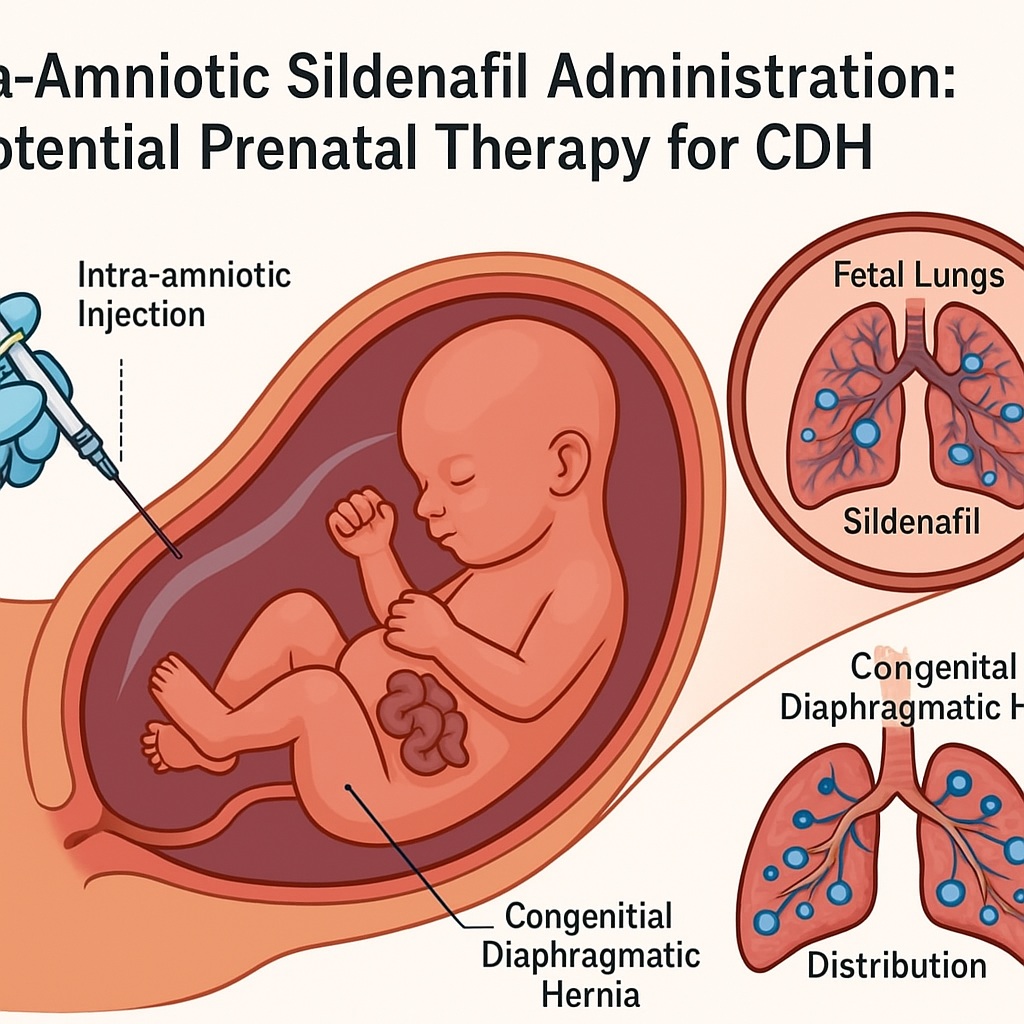
Among the potential therapeutic agents under investigation, sildenafil citrate, a potent phosphodiesterase-5 (PDE-5) inhibitor, has garnered exceptional attention for its vasodilatory and cytoprotective effects. Traditionally employed in the management of erectile dysfunction and pulmonary arterial hypertension, sildenafil’s mechanism—enhancing cyclic guanosine monophosphate (cGMP) signaling—offers unique benefits in conditions characterized by vascular remodeling and hypoplasia, such as congenital diaphragmatic hernia (CDH).
The study titled “Intra-amniotic sildenafil administration in rabbits: Safety, pharmacokinetics, organ distribution, and histologic evaluation” represents a major step forward in fetal pharmacotherapy. By delivering sildenafil directly into the amniotic cavity of pregnant rabbits, researchers sought to overcome the limitations of maternal drug delivery, determine fetal pharmacokinetics, and assess the safety and tissue distribution of this intervention.
The findings reveal not only the feasibility of intra-amniotic (IA) drug delivery, but also the potential of sildenafil as a prenatal therapy for pulmonary hypoplasia associated with CDH—one of the most challenging congenital malformations in perinatal medicine.
Congenital Diaphragmatic Hernia and the Rationale for Prenatal Sildenafil Therapy
Congenital diaphragmatic hernia (CDH) is a structural anomaly characterized by incomplete formation of the diaphragm, allowing abdominal organs to herniate into the thoracic cavity. This mechanical intrusion compresses developing lungs, resulting in pulmonary hypoplasia and persistent pulmonary hypertension of the newborn (PPHN). Despite advancements in neonatal intensive care, extracorporeal membrane oxygenation (ECMO), and surgical repair, mortality rates remain as high as 30–50% in severe cases.
The underlying pathophysiology extends beyond simple anatomical compression. Experimental evidence shows impaired pulmonary vascular development, smooth muscle hyperplasia, and aberrant nitric oxide–cGMP signaling in CDH. The resulting high pulmonary vascular resistance leads to right-to-left shunting and severe hypoxemia postnatally.
Herein lies the pharmacological logic for sildenafil. As a PDE-5 inhibitor, sildenafil prevents cGMP degradation, thereby enhancing nitric oxide–mediated vasodilation. In fetal models, increased cGMP availability has been shown to promote vascular relaxation, alveolar growth, and angiogenesis—all key deficits in CDH-associated pulmonary hypoplasia.
Traditional maternal sildenafil therapy, while conceptually promising, suffers from two major limitations:
- Limited transplacental transfer: The placenta acts as a partial barrier, reducing fetal drug exposure.
- Maternal side effects: Systemic vasodilation may lead to hypotension or impaired uteroplacental perfusion.
The intra-amniotic route circumvents both issues by delivering sildenafil directly to the fetal compartment, ensuring localized exposure while minimizing maternal risk. The rabbit study provides a crucial proof-of-concept for this approach.
Experimental Design: A Model of Translational Fetal Pharmacology
The investigators employed a pregnant rabbit model, a species known for its well-characterized placentation and fetal physiology, which closely mirror certain aspects of human gestation. Intra-amniotic injections were performed under ultrasound guidance on gestational day 27, corresponding to late gestation when fetal pulmonary development parallels the canalicular–saccular stages in humans.
Two primary objectives guided the study:
- To assess the pharmacokinetics and tissue distribution of sildenafil following intra-amniotic administration.
- To evaluate maternal and fetal safety, including potential histological alterations in major organs.
Amniotic fluid, maternal plasma, and fetal tissues (lung, liver, brain, kidney) were sampled at various intervals post-injection (0.5–8 hours). Sildenafil and its active metabolite, N-desmethylsildenafil, were quantified using liquid chromatography–mass spectrometry (LC–MS), providing high-resolution pharmacokinetic profiling.
Histological evaluations of fetal and maternal organs were conducted using hematoxylin–eosin staining, searching for inflammatory, necrotic, or degenerative changes attributable to sildenafil exposure.
This methodical design represents an exemplary intersection of pharmacology, toxicology, and fetal medicine, setting a precedent for preclinical evaluation of intra-amniotic therapeutics.
Pharmacokinetics: Rapid Absorption and Targeted Fetal Exposure
The pharmacokinetic data demonstrated rapid absorption of sildenafil from the amniotic cavity into the fetal circulation. Detectable levels appeared within 30 minutes post-administration, peaking around 2 hours, and declining thereafter. This kinetic profile aligns with the physiological swallowing and respiratory-like movements of the fetus, which facilitate trans-amniotic absorption through the gastrointestinal tract and pulmonary epithelia.
Interestingly, fetal sildenafil concentrations significantly exceeded maternal plasma levels, confirming minimal maternal systemic exposure—a critical safety milestone. The fetal lung and liver showed the highest drug accumulation, reflecting both the target organ for therapeutic effect (lungs) and the primary site of metabolism (liver).
The elimination half-life of sildenafil in fetal tissues was modestly prolonged compared to adult profiles, likely reflecting immature hepatic metabolism and lower cytochrome P450 activity in the developing fetus. This prolonged retention may enhance pharmacodynamic benefits but necessitates precise dose optimization to avoid accumulation and toxicity.
Moreover, the detection of N-desmethylsildenafil—a potent PDE-5 inhibitor in its own right—demonstrates active metabolic transformation within fetal tissues, suggesting that the fetal liver is already capable of biotransformation, a crucial factor for therapeutic efficacy.
Safety Profile: Absence of Maternal and Fetal Toxicity
The cornerstone of any prenatal therapeutic approach is safety—for both the mother and the fetus. Histopathological examination revealed no evidence of tissue damage in maternal or fetal organs following intra-amniotic sildenafil administration.
Specifically:
- Fetal lungs maintained normal alveolar architecture with no signs of edema or inflammatory infiltration.
- Hepatic and renal tissues displayed intact parenchymal organization without vacuolization or necrosis.
- Maternal uterus and placenta remained morphologically normal, with no inflammatory or degenerative alterations.
No changes in maternal behavior, appetite, or weight gain were observed throughout the experimental period. These results establish that intra-amniotic sildenafil is well tolerated and non-toxic at therapeutic concentrations—a crucial finding in the development of prenatal pharmacotherapy.
Importantly, the absence of histologic abnormalities in fetal brain tissue also mitigates concerns regarding potential neurotoxicity or PDE-5–related neuronal signaling disruptions. This aligns with prior data showing that sildenafil poorly penetrates the fetal blood–brain barrier, providing an added layer of neurological safety.
Organ Distribution: Targeting the Lungs Where It Matters Most
Quantitative tissue analysis revealed that fetal lungs exhibited the highest sildenafil concentration, followed by the liver and kidneys. This selective accumulation underscores the therapeutic precision achievable through intra-amniotic delivery. The mechanism likely involves direct exposure of the respiratory epithelium to amniotic fluid, allowing diffusion into the pulmonary parenchyma—precisely where vasodilatory and angiogenic effects are needed in CDH.
This localized pharmacological targeting holds particular promise for fetal pulmonary hypoplasia, where promoting vascular growth and alveolar maturation prior to birth could significantly enhance postnatal lung function. By boosting endothelial nitric oxide signaling and reducing vascular smooth muscle proliferation, sildenafil may preempt the structural and functional hallmarks of CDH-associated pulmonary hypertension.
Moreover, the limited accumulation in non-target organs suggests a favorable therapeutic index, reducing systemic fetal exposure and potential off-target effects.
Translational Relevance: A Step Toward Prenatal Pulmonary Rescue
The broader implications of these findings extend to the prenatal management of CDH, a field where intervention timing and method are critical. Current fetal therapies, such as fetoscopic endoluminal tracheal occlusion (FETO), aim to stimulate lung growth mechanically by trapping fluid within the lungs. While FETO improves survival in severe CDH, it is technically demanding and associated with preterm labor and procedural complications.
In contrast, pharmacological strategies like intra-amniotic sildenafil represent a non-invasive, molecular approach to the same goal—enhancing pulmonary development without mechanical obstruction. The ability to induce vasodilation, stimulate angiogenesis, and improve endothelial function pharmacologically may complement or even replace existing surgical modalities in the future.
Furthermore, the pharmacokinetic and safety data from this rabbit model pave the way for dose translation studies, fetal pharmacodynamics modeling, and eventually human clinical trials. Given sildenafil’s extensive safety data in adults and neonates, its repositioning for intrauterine use is not only scientifically rational but also ethically feasible under controlled research protocols.
Challenges and Considerations for Clinical Translation
While the experimental findings are promising, several translational challenges must be addressed before intra-amniotic sildenafil can be implemented clinically.
First, species differences in placental structure, fetal metabolism, and amniotic fluid dynamics limit direct extrapolation of dosing regimens from rabbits to humans. Human fetuses exhibit slower amniotic turnover and distinct drug absorption kinetics, necessitating detailed pharmacometric modeling.
Second, the long-term safety of in utero sildenafil exposure remains uncertain. Although no short-term toxicity was observed, potential effects on vascular development, endocrine regulation, or postnatal pulmonary adaptation warrant longitudinal follow-up in animal models before clinical translation.
Third, the timing of administration is crucial. Pulmonary vasculature and alveolar structures undergo stage-dependent maturation. Determining the optimal gestational window for intervention—likely between 24 and 30 weeks in humans—will be key to maximizing therapeutic efficacy without disrupting normal organogenesis.
Lastly, delivery technique requires refinement. While ultrasound-guided intra-amniotic injection is feasible, repeated dosing or controlled-release formulations may improve pharmacologic consistency and reduce procedural risk. Development of biodegradable nanoparticles or hydrogels capable of sustained sildenafil release within the amniotic fluid could represent the next technological leap.
Mechanistic Insight: Beyond Vasodilation
Although sildenafil’s vasodilatory effect via PDE-5 inhibition is well established, emerging evidence points to broader cytoprotective and developmental roles that may be particularly relevant in the fetal lung.
- Angiogenic stimulation: Sildenafil upregulates VEGF and eNOS expression, fostering capillary formation essential for alveolar development.
- Anti-apoptotic signaling: By elevating intracellular cGMP, sildenafil mitigates apoptosis in pulmonary endothelial cells, preserving tissue integrity.
- Antioxidant modulation: Enhanced cGMP signaling counteracts oxidative stress—a key factor in CDH-associated vascular remodeling.
These multifaceted actions suggest that sildenafil may function not merely as a symptomatic vasodilator but as a developmental modulator, capable of reshaping the pulmonary microenvironment during gestation.
Ethical and Regulatory Implications
Any prenatal intervention involving direct fetal drug administration raises profound ethical considerations. The principle of maternal–fetal dual patienthood demands that therapeutic benefits to the fetus do not come at undue maternal risk. The intra-amniotic route, by minimizing systemic maternal exposure, aligns well with this principle.
However, the path to regulatory approval will require:
- Comprehensive reproductive toxicology data across multiple species.
- Assessment of developmental endpoints postnatally, including growth, neurodevelopment, and fertility.
- Compliance with Good Laboratory and Clinical Practice (GLP/GCP) standards in future trials.
If successfully validated, intra-amniotic sildenafil could inaugurate a new era of targeted fetal pharmacotherapy, balancing efficacy, safety, and ethical responsibility.
Future Perspectives: Toward Precision Fetal Medicine
The concept of precision fetal therapy envisions interventions tailored to specific genetic, structural, and physiological conditions of the fetus. Intra-amniotic sildenafil fits squarely within this vision. By combining molecular imaging, fetal echocardiography, and quantitative pharmacokinetics, clinicians could one day individualize sildenafil therapy based on fetal lung size, Doppler flow parameters, and genetic biomarkers of pulmonary growth.
Moreover, the approach could extend beyond CDH to other conditions characterized by fetal pulmonary hypertension, including oligohydramnios, intrauterine growth restriction (IUGR), and twin-to-twin transfusion syndrome. In each scenario, localized sildenafil delivery could mitigate vascular remodeling and improve perinatal outcomes.
The rabbit study thus represents more than a pharmacological experiment—it marks the dawn of intra-amniotic drug therapeutics as a legitimate branch of perinatal medicine.
Conclusion
The intra-amniotic administration of sildenafil in rabbits stands as a landmark achievement in fetal pharmacology. It confirms that targeted fetal exposure can be achieved safely, effectively, and selectively through the amniotic route. The demonstration of rapid absorption, favorable tissue distribution, and absence of toxicity provides the foundational data necessary for translational advancement.
For congenital diaphragmatic hernia—a condition historically defined by high mortality and limited prenatal options—this approach offers hope. By harnessing sildenafil’s vasodilatory, angiogenic, and cytoprotective capacities, intra-amniotic therapy may one day transform fetal pulmonary hypoplasia from a fatal diagnosis into a manageable condition.
The road from rabbit models to human clinical application remains long and cautious, but the direction is clear. Intra-amniotic sildenafil may soon join the ranks of pioneering prenatal interventions that redefine what is possible in the realm of fetal medicine and developmental therapeutics.
FAQ: Intra-Amniotic Sildenafil and CDH
1. Why use sildenafil for congenital diaphragmatic hernia?
Sildenafil enhances nitric oxide–cGMP signaling, promoting pulmonary vasodilation and vascular growth. In CDH, where pulmonary hypoplasia and hypertension are major complications, sildenafil may improve fetal lung development and postnatal adaptation.
2. How does intra-amniotic administration differ from maternal dosing?
Maternal sildenafil must cross the placenta, often resulting in subtherapeutic fetal levels and maternal side effects. Intra-amniotic administration delivers the drug directly to the fetal compartment, achieving higher lung concentrations with minimal maternal exposure.
3. Is intra-amniotic sildenafil safe for the mother and fetus?
In the rabbit model, no histologic abnormalities or toxic effects were observed in either maternal or fetal tissues. While these results are encouraging, long-term safety and efficacy in humans will require rigorous clinical evaluation.