Introduction
Erectile and reproductive dysfunctions are among the most common physiological manifestations of oxidative stress in men. The male reproductive system—particularly the testes and penile tissues—requires a finely balanced redox environment for the optimal production of testosterone, the maintenance of spermatogenesis, and the orchestration of the nitric oxide (NO) signaling cascade essential for erection. When this equilibrium falters, the result is not merely biochemical chaos but a measurable decline in vitality, fertility, and confidence.
While synthetic drugs such as sildenafil and tadalafil provide rapid symptomatic relief, they do not address the underlying pathophysiological imbalance—oxidative damage, hormonal dysregulation, and endothelial inflammation. This has reinvigorated scientific interest in phytotherapeutics, where plants traditionally known for their aphrodisiac properties are now being investigated for their molecular and biochemical effects on male sexual health.
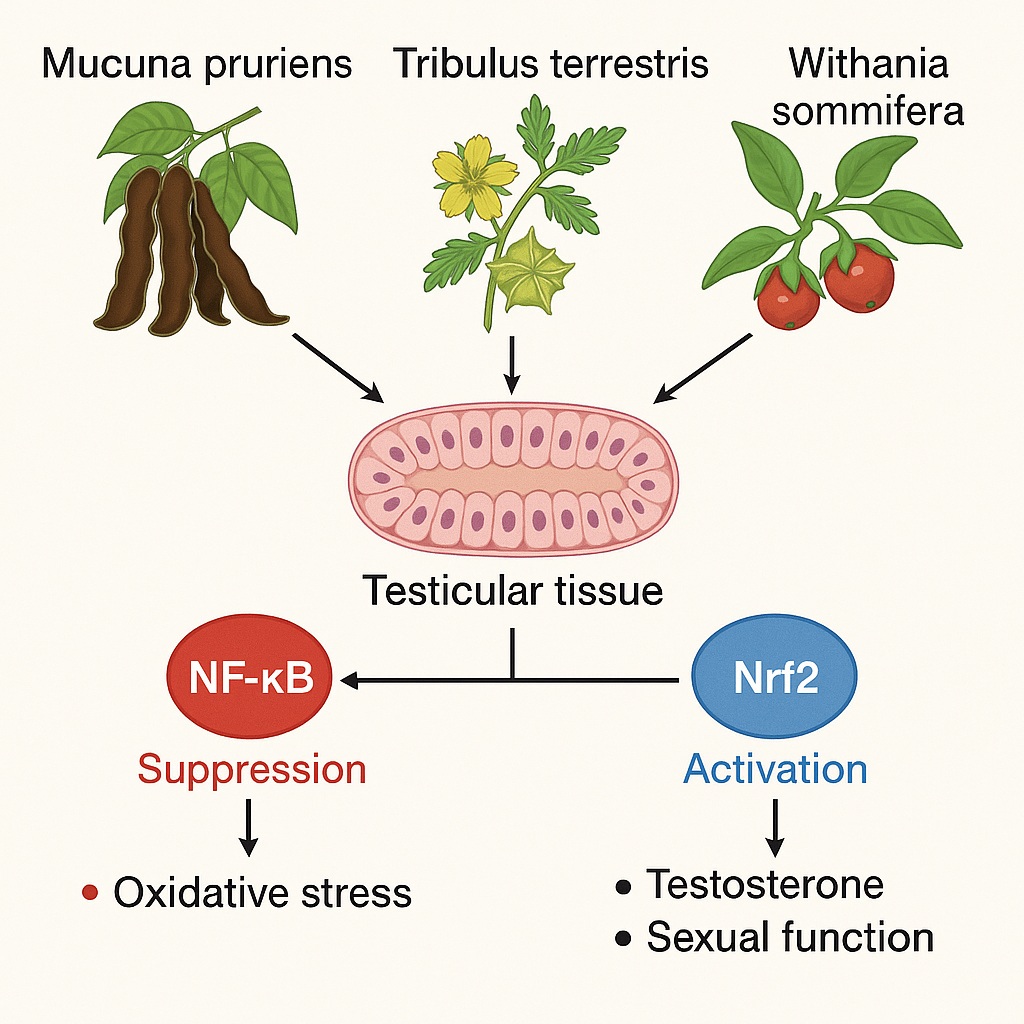
The study under discussion provides an elegant comparative framework, examining the effects of Mucuna pruriens, Tribulus terrestris, and Withania somnifera (Ashwagandha) on male rats with impaired sexual performance, through the lens of two major regulatory pathways: NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) and Nrf2 (nuclear factor erythroid 2–related factor 2). Together, these transcriptional regulators form a seesaw of oxidative balance: one drives inflammation, the other defends against it. Understanding their interplay under herbal modulation may illuminate how natural compounds can restore not just virility, but cellular harmony.
The Antioxidant–Aphrodisiac Connection
Oxidative Stress as the Saboteur of Male Sexual Health
Male reproductive physiology is an oxidative paradox: it depends on reactive oxygen species (ROS) for sperm capacitation and acrosomal reaction, yet excessive ROS leads to lipid peroxidation, DNA fragmentation, and germ cell apoptosis. The testes and penile endothelial cells are particularly susceptible due to their high polyunsaturated fatty acid content and rich mitochondrial activity.
When oxidative stress overwhelms the antioxidant defense system, the resulting damage affects both Leydig cells, responsible for testosterone synthesis, and endothelial cells, which generate nitric oxide for vasodilation. Consequently, both sperm quality and erectile response decline.
The NF-κB pathway, a master regulator of inflammatory gene expression, is typically overactivated under such oxidative conditions. In contrast, Nrf2—often described as the “guardian of redox homeostasis”—controls the expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase. When Nrf2 is repressed and NF-κB overexpressed, a cascade of cellular dysfunction unfolds, culminating in reproductive impairment.
Phytotherapy: The Natural Counterbalance
Herbal extracts have long been proposed to counteract this oxidative burden. Beyond their folkloric reputations as libido enhancers, plants such as Mucuna pruriens, Tribulus terrestris, and Withania somnifera contain bioactive compounds—flavonoids, alkaloids, saponins, and withanolides—that can scavenge ROS, upregulate antioxidant genes, and restore hormonal equilibrium.
This comparative study sought not merely to affirm their aphrodisiac potential but to quantify their molecular impact—to determine whether these herbs could modulate oxidative-inflammatory cross talk by influencing the Nrf2 and NF-κB signaling pathways.
The Herbal Contenders: Pharmacologic Background
Mucuna pruriens (Velvet Bean)
Mucuna pruriens is a leguminous plant celebrated for its high content of L-DOPA, the precursor of dopamine. Dopaminergic signaling enhances sexual motivation and penile erection via central nervous system pathways involving the hypothalamus and pituitary. Additionally, M. pruriens is rich in alkaloids, phenolics, and flavonoids that contribute to antioxidant defense and spermatogenic stimulation.
Earlier studies have shown that M. pruriens restores seminal plasma antioxidant levels and testosterone concentration in infertile men, indicating a dual neural and endocrine mechanism.
Tribulus terrestris (Puncture Vine)
Tribulus terrestris owes its aphrodisiac reputation to its protodioscin, a steroidal saponin hypothesized to increase androgen receptor density and modulate nitric oxide release in cavernous tissue. Despite some controversies regarding its human efficacy, preclinical data consistently show improved mount latency, intromission frequency, and sperm motility in treated male animals.
Its pharmacological potential lies in endothelial nitric oxide synthase (eNOS) upregulation and lipid peroxidation inhibition, linking it directly to redox balance and vascular function.
Withania somnifera (Ashwagandha)
Withania somnifera is perhaps the most thoroughly investigated adaptogen in Ayurvedic medicine. Its withanolides and sitoindosides modulate stress hormone pathways (cortisol reduction) while exerting potent antioxidant and anti-inflammatory effects. Clinically, Ashwagandha has demonstrated testosterone elevation, improved semen parameters, and reduced stress-induced infertility.
Its primary mechanism is believed to involve upregulation of Nrf2, thereby activating downstream antioxidant enzymes and reducing oxidative load across multiple organ systems.
Mechanistic Pathways: NF-κB and Nrf2 Crosstalk
The choice to evaluate both NF-κB and Nrf2 pathways in this study reflects an appreciation for the biological dualism governing oxidative stress and inflammation. The two systems are interconnected through shared redox-sensitive intermediates.
NF-κB: The Inflammatory Driver
NF-κB resides in the cytoplasm under basal conditions, bound to its inhibitor IκB. Upon oxidative or inflammatory stimulus, IκB is phosphorylated and degraded, allowing NF-κB to translocate to the nucleus, where it upregulates pro-inflammatory genes such as TNF-α, IL-6, and inducible nitric oxide synthase (iNOS).
Chronic activation of NF-κB in the testes or penile tissue results in inflammation-induced vascular dysfunction and Leydig cell apoptosis, ultimately depressing testosterone synthesis.
Nrf2: The Antioxidant Defender
Nrf2, conversely, remains sequestered by its repressor Keap1 under normal conditions. Oxidative stress induces conformational changes in Keap1, freeing Nrf2 to migrate into the nucleus and bind to antioxidant response elements (ARE) in DNA. This transcriptionally activates genes encoding heme oxygenase-1 (HO-1), glutathione S-transferase (GST), and NAD(P)H:quinone oxidoreductase (NQO1).
Through these actions, Nrf2 restores redox equilibrium, inhibits NF-κB activation indirectly, and protects against testicular oxidative damage.
The Interplay
The crucial insight from this study lies in observing that herbal extracts can shift the balance between these two pathways. A simultaneous downregulation of NF-κB and upregulation of Nrf2 signifies restoration of cellular homeostasis—a molecular reflection of the improved sexual behavior and fertility indices observed in treated rats.
Experimental Design and Key Findings
Study Overview
Adult male rats were divided into control and treatment groups, receiving standardized doses of the three extracts. Behavioral assays measured mount latency (ML), intromission latency (IL), ejaculation latency (EL), and post-ejaculatory interval (PEI)—classic indices of sexual performance. Biochemical assays quantified testosterone, lipid peroxidation (MDA), antioxidant enzyme activities, and mRNA expression levels of NF-κB and Nrf2.
Behavioral Outcomes
All three extracts improved sexual behavior parameters, albeit with differing magnitudes. The most pronounced effects were observed with Mucuna pruriens, which significantly shortened ML and IL, increased copulatory frequency, and enhanced ejaculation latency, indicating heightened libido and endurance.
Tribulus terrestris improved erectile response modestly, while Withania somnifera primarily affected post-ejaculatory recovery, consistent with its adaptogenic, stress-modulating profile.
Biochemical Findings
Across all groups, antioxidant enzyme activities (SOD, CAT, GPx) increased significantly relative to control, while MDA levels—a marker of lipid peroxidation—declined. Mucuna pruriens induced the greatest elevation in testicular testosterone concentration, correlating strongly with behavioral enhancement.
At the molecular level, the extracts downregulated NF-κB and upregulated Nrf2 expression, validating the hypothesis that their effects extend beyond mere hormonal stimulation to include direct modulation of redox-sensitive transcriptional programs.
Comparative Pharmacodynamics of the Extracts
Mucuna pruriens: The Dopaminergic Antioxidant
M. pruriens displayed the most robust profile, combining central dopaminergic stimulation (via L-DOPA) with peripheral antioxidant effects. Its capacity to activate Nrf2 while suppressing NF-κB reflects its biochemical versatility—restoring both neural drive and testicular oxidative balance. The extract also elevated testosterone and improved sperm parameters, suggesting enhanced Leydig cell viability and steroidogenic efficiency.
Tribulus terrestris: The Endothelial Modulator
Tribulus terrestris appeared to act primarily through vascular mechanisms, augmenting NO bioavailability and reducing oxidative endothelial damage. While its Nrf2 activation was moderate compared to M. pruriens, it effectively suppressed NF-κB, indicating anti-inflammatory benefits that may contribute to sustained erectile function.
However, its testosterone enhancement was less pronounced, suggesting its utility might lie more in erectile modulation than endocrine correction.
Withania somnifera: The Stress-Modulated Rebalancer
Withania somnifera demonstrated significant upregulation of Nrf2 with only modest downregulation of NF-κB. This pattern aligns with its adaptogenic role—reducing cortisol, alleviating oxidative load, and thereby indirectly improving reproductive function. It may not directly stimulate sexual behavior but rather enhances resilience, making it valuable in stress-induced or psychogenic infertility.
Molecular Integration: The NF-κB–Nrf2 Synergy
This comparative framework highlights an elegant molecular truth: sexual health is a redox-dependent process, and herbal aphrodisiacs function less as “libido potions” and more as biochemical rebalancers.
The observed effects suggest a model in which:
- Mucuna pruriens activates dopaminergic pathways and Nrf2-mediated antioxidant response.
- Tribulus terrestris improves vascular NO signaling and suppresses NF-κB-driven inflammation.
- Withania somnifera reinforces systemic stress tolerance via Nrf2 activation and cortisol reduction.
Together, these extracts converge on a single outcome: restoration of testicular and endothelial redox homeostasis, leading to normalized hormonal synthesis and improved sexual function.
Clinical and Translational Implications
Toward Human Applications
While extrapolation from rodents to humans demands caution, the pathways involved are evolutionarily conserved. The findings reinforce the premise that phytochemicals targeting NF-κB and Nrf2 can form the molecular foundation of next-generation nutraceuticals or adjunct therapies for male infertility and erectile dysfunction.
Such therapies may offer dual-action benefits—modulating both oxidative stress and inflammatory signaling—where synthetic PDE5 inhibitors act merely as symptomatic vasodilators.
Synergy, Not Substitution
It is crucial to emphasize that these herbs are not direct pharmacologic substitutes for conventional therapy. Instead, they may serve as synergistic adjuncts, improving endothelial responsiveness, enhancing testosterone synthesis, and protecting against the oxidative sequelae of chronic stress, metabolic syndrome, or aging.
Precision Phytotherapy
The future of such interventions likely lies in standardized, pathway-specific formulations—combining bioactive compounds in concentrations that selectively activate Nrf2 while tempering NF-κB. Advanced bioassays, such as transcriptomic profiling of patient-derived cells, could guide personalized herbal prescriptions, transforming traditional remedies into precision phytotherapy.
Conclusion
This comparative study does more than validate traditional aphrodisiacs—it maps their molecular choreography. Through concurrent modulation of the NF-κB–Nrf2 axis, Mucuna pruriens, Tribulus terrestris, and Withania somnifera reestablish the biochemical harmony that underpins male sexual function.
The real insight lies not in the enhancement of libido per se, but in the reversal of oxidative pathology—in restoring the cellular environment required for hormonal synthesis, endothelial function, and reproductive vitality. The message is clear: sexual health is not just hormonal; it is redox-regulated, and nature’s pharmacy has long held the keys to recalibration.
FAQ
1. How do these herbal extracts improve male sexual function at the molecular level?
They modulate redox-sensitive transcription factors. Specifically, they suppress NF-κB (reducing inflammation) and activate Nrf2 (enhancing antioxidant defenses). This rebalances oxidative stress, improves testosterone synthesis, and restores vascular function critical for erection and fertility.
2. Which herbal extract showed the strongest aphrodisiac effect?
Mucuna pruriens produced the most comprehensive improvement—enhancing libido, testosterone, sperm quality, and oxidative defense—due to its combined dopaminergic stimulation and Nrf2 activation.
3. Can these findings be applied directly to human treatments?
Not directly, but they form a strong scientific basis for developing standardized nutraceuticals or herbal adjunct therapies. Human clinical trials are needed to confirm efficacy, safety, and optimal dosing.