Introduction
Modern medicine often suffers from compartmentalization: cardiologists manage the heart, endocrinologists handle hormones, and urologists concern themselves with the prostate, kidneys, and male sexual function. Yet the biological truth does not respect these professional boundaries. Erectile dysfunction (ED), once viewed purely as a urological or psychological inconvenience, is now recognized as a powerful harbinger of systemic disease, particularly cardiovascular disease (CVD).
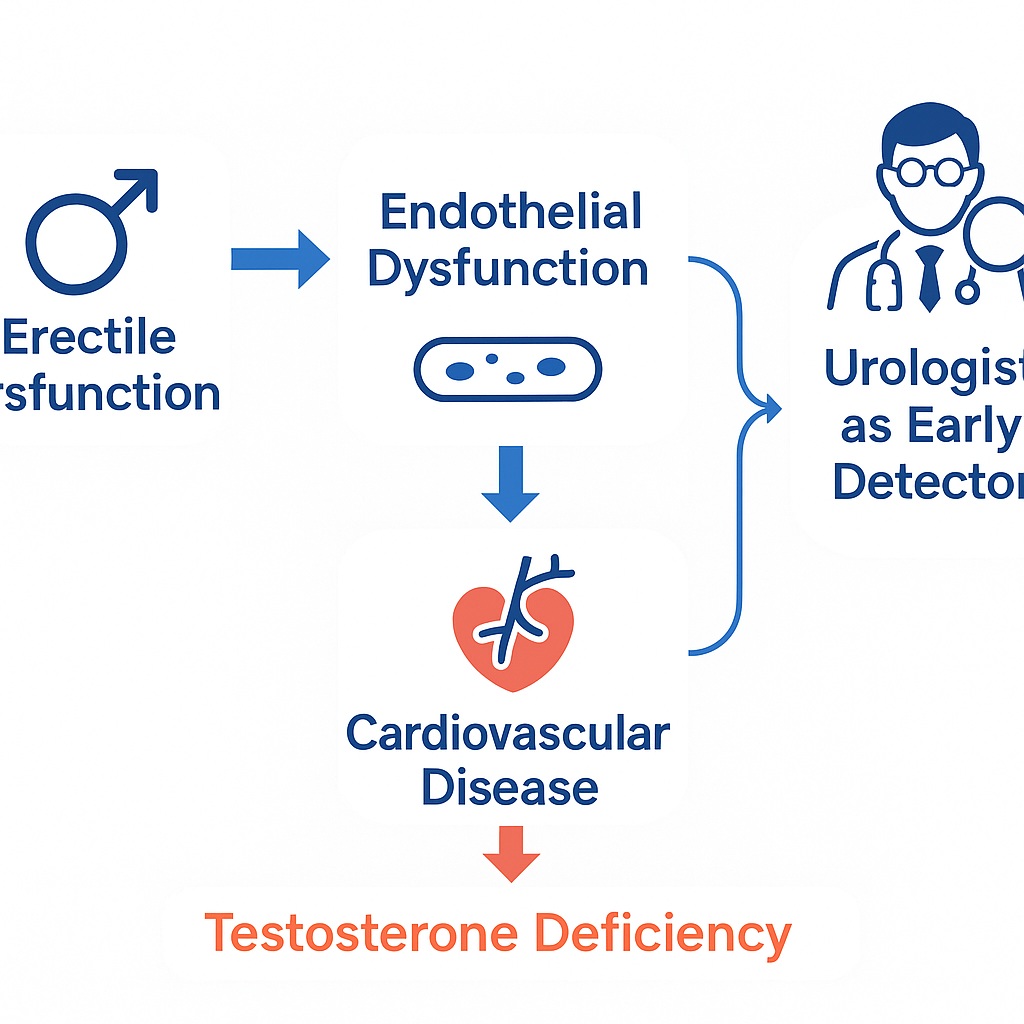
ED and CVD are two faces of the same vascular coin, united by a common denominator: endothelial dysfunction. What appears in the penis as difficulty achieving erections often reflects the earliest signs of compromised vascular health throughout the arterial tree. In this sense, the penis, with its exquisitely small-caliber arteries, functions as a biological “canary in the coal mine,” signaling cardiovascular risk years before the first myocardial infarction or stroke.
Urologists, positioned at this crossroads of reproductive and systemic health, are uniquely equipped to identify these early warning signs. But to fulfill this role, the discipline must embrace a broader perspective, integrating cardiometabolic evaluation into the diagnostic workup of ED. In doing so, urologists can become not merely specialists in sexual function but guardians of men’s long-term cardiovascular health.
Erectile Dysfunction as an Early Marker of Cardiovascular Disease
The Massachusetts Male Aging Study provided one of the earliest epidemiological wake-up calls. Men with ED were found to carry a significantly higher burden of cardiovascular risk factors, including hypertension, dyslipidemia, obesity, and diabetes. Importantly, ED often preceded cardiovascular events by several years, serving as an opportunity window for preventive intervention.
The underlying mechanism is endothelial dysfunction. Normally, the vascular endothelium orchestrates vasodilation, platelet regulation, and anti-inflammatory control through nitric oxide (NO) release. In metabolic syndrome and diabetes, however, NO bioavailability declines due to oxidative stress and lipid abnormalities. The earliest clinical manifestation may not be angina but rather difficulty sustaining erections.
By recognizing ED as a sentinel marker, urologists can pivot from reactive treatment to proactive prevention. This means that each consultation for sexual dysfunction should be viewed not only as a conversation about intimacy but also as a screening opportunity for hypertension, diabetes, obesity, and dyslipidemia.
Testosterone, Endothelium, and Sexual Function
For decades, testosterone was pigeonholed as the hormone of libido, exerting influence on sexual desire rather than erectile mechanics. This reductionist view has been decisively overturned. Testosterone receptors are widely expressed in penile smooth muscle and endothelial cells, where the hormone regulates apoptosis, smooth muscle tone, and nitric oxide synthase expression.
Declining testosterone impairs both the structural and functional substrate of erectile capacity. Hypogonadal men demonstrate loss of nocturnal erections, impaired cavernosal hemodynamics, and increased adipocyte infiltration within the corpus cavernosum—a precursor to veno-occlusive dysfunction. These defects are partly reversible with androgen replacement, reinforcing testosterone’s critical role in penile health.
Moreover, testosterone deficiency correlates with endothelial dysfunction systemically. Low levels are linked with impaired vasodilation, higher inflammatory markers, and greater arterial stiffness, suggesting that androgen decline contributes not only to ED but also to the pathogenesis of atherosclerosis. Thus, the connection between testosterone and vascular health is not coincidental but mechanistically intertwined.
Metabolic Syndrome, Diabetes, and the Testosterone Connection
The metabolic syndrome—defined by central obesity, hypertension, dyslipidemia, and insulin resistance—constitutes fertile ground for both ED and CVD. Testosterone deficiency is both a contributor to and a consequence of this syndrome, creating a vicious cycle.
Adipose tissue secretes inflammatory cytokines and leptin, both of which suppress testicular steroidogenesis. Hyperinsulinemia further inhibits sex hormone-binding globulin, lowering bioavailable testosterone. Conversely, low testosterone exacerbates visceral adiposity, worsens insulin resistance, and accelerates progression toward type 2 diabetes and cardiovascular disease.
Clinical studies confirm these bidirectional links. Men with low total or free testosterone exhibit higher risks of metabolic syndrome and type 2 diabetes. Conversely, androgen deprivation therapy for prostate cancer produces rapid-onset metabolic derangements: increased fat mass, elevated insulin, and worsening lipid profiles. These findings illustrate testosterone’s pivotal role in metabolic homeostasis—and why ignoring androgen status in men with ED is a missed opportunity for systemic health preservation.
The Urologist’s Expanding Role in Cardiovascular Prevention
When a man presents with erectile complaints, the urologist stands at a diagnostic fork in the road. One path is narrowly focused: prescribe a PDE5 inhibitor, perhaps reassure the patient, and move on. The other path is integrative: recognize ED as a vascular alarm bell, investigate cardiometabolic risks, and collaborate with internists and cardiologists. The latter approach is not only scientifically justified but ethically imperative.
Practical steps for urologists include:
- Routine cardiovascular risk assessment: blood pressure, fasting glucose, lipid panel, waist circumference, and BMI.
- Hormonal profiling: measure total and free testosterone, SHBG, and rule out hypogonadism.
- Lifestyle counseling: encourage weight loss, exercise, and smoking cessation, all of which improve both erectile and cardiovascular outcomes.
Such interventions reframe ED consultations into holistic men’s health evaluations. In this model, the urologist becomes both a specialist and a general health sentinel.
Therapeutic Synergies: PDE5 Inhibitors and Testosterone
Phosphodiesterase type 5 inhibitors (PDE5Is), such as sildenafil and tadalafil, have revolutionized ED management. Yet up to one-third of patients fail to respond. One underappreciated reason is testosterone deficiency.
Androgen deprivation downregulates PDE5 expression in penile tissue, leaving PDE5Is with little substrate to act upon. Restoring testosterone enhances PDE5 expression and nitric oxide synthase activity, thereby improving responsiveness to PDE5Is. Clinical studies demonstrate that combined therapy with testosterone and PDE5Is rescues erectile function in previously unresponsive hypogonadal men.
This pharmacological synergy underscores a central principle: treating ED effectively often requires addressing both the vascular signaling pathway (via PDE5Is) and the hormonal foundation (via testosterone). Ignoring either element risks therapeutic failure.
Testosterone Replacement in Metabolic and Cardiovascular Disease
The once-prevailing fear that testosterone therapy would worsen cardiovascular outcomes has largely been debunked. Contemporary evidence suggests the opposite: low testosterone is associated with higher cardiovascular mortality, while testosterone replacement improves risk profiles in hypogonadal men.
Randomized controlled trials show that testosterone therapy reduces visceral fat, improves insulin sensitivity, lowers fasting glucose, and favorably alters lipid profiles. In men with type 2 diabetes, testosterone supplementation enhances glycemic control and reduces markers of inflammation. Long-term studies indicate improvements in waist circumference, lean body mass, and metabolic syndrome severity.
While not a panacea, testosterone therapy represents a legitimate cardiovascular risk-reducing intervention in appropriately selected men. Urologists, as gatekeepers of androgen evaluation, play a critical role in identifying candidates who stand to benefit from such therapy.
Conclusion
Erectile dysfunction is no longer a purely sexual complaint. It is a clinical signpost pointing toward systemic vascular pathology, metabolic derangements, and hormonal insufficiency. Testosterone, once relegated to the realm of libido, has reemerged as a hormone of systemic relevance, influencing glucose control, lipid metabolism, vascular integrity, and bone and muscle strength.
For the urologist, the implications are profound. By expanding their lens from the genital to the systemic, urologists can detect cardiovascular disease in its earliest, most treatable stages. They can integrate hormonal and vascular insights, turning each ED consultation into a platform for preventive cardiology.
In the end, the convergence of ED, testosterone, and cardiovascular disease offers an opportunity. Not only to restore sexual function but to save lives by preventing heart attacks, strokes, and diabetic complications. The penis may be a small organ, but as a diagnostic gateway, it speaks volumes about the state of the vascular tree.
FAQ
1. Why is erectile dysfunction considered a warning sign for cardiovascular disease?
Because ED often results from endothelial dysfunction—the same process underlying atherosclerosis. The small penile arteries manifest symptoms earlier than coronary or carotid vessels, making ED an early indicator of systemic vascular disease.
2. How does testosterone deficiency contribute to both ED and cardiovascular risk?
Low testosterone impairs penile smooth muscle structure, reduces nitric oxide signaling, and promotes adiposity and insulin resistance. This dual effect accelerates both erectile dysfunction and the metabolic syndrome, increasing cardiovascular risk.
3. Should testosterone replacement therapy be considered safe for men with cardiovascular disease?
Current evidence suggests that testosterone therapy, when appropriately indicated and monitored, improves metabolic and cardiovascular profiles. It should be prescribed judiciously, with careful attention to prostate health and hematocrit levels, but it is not inherently unsafe.