Introduction
Sildenafil citrate, known worldwide under its brand name Viagra, revolutionized the management of male erectile dysfunction when it entered clinical practice in the late 1990s. Its mechanism of action is well established: inhibition of phosphodiesterase type 5 (PDE5) leads to elevated cyclic guanosine monophosphate (cGMP) levels, facilitating smooth muscle relaxation and penile erection. While its therapeutic role is undeniable, the widespread use of sildenafil has prompted questions beyond its immediate vascular effects, particularly regarding safety in populations outside its initial target group.
One such concern involves reproductive health. Sildenafil is frequently used by men of reproductive age, both for medical and recreational reasons. Beyond this, it has been studied experimentally for conditions such as pulmonary hypertension, intrauterine growth restriction (IUGR), and preterm labor. These expanding indications demand a closer look at the developmental and reproductive safety of the drug. Animal studies, including controlled experiments in mice, provide valuable insight into whether sildenafil poses risks to embryos and fetuses when exposure occurs during pregnancy.
This article examines in detail the findings from developmental toxicity studies in SWR/J mice, explores possible mechanisms of embryonic and fetal effects, and discusses the implications for clinical use in humans. While sildenafil remains a cornerstone of sexual medicine, understanding its potential limitations is critical to ensure safe and responsible application.
Experimental Models and Study Design
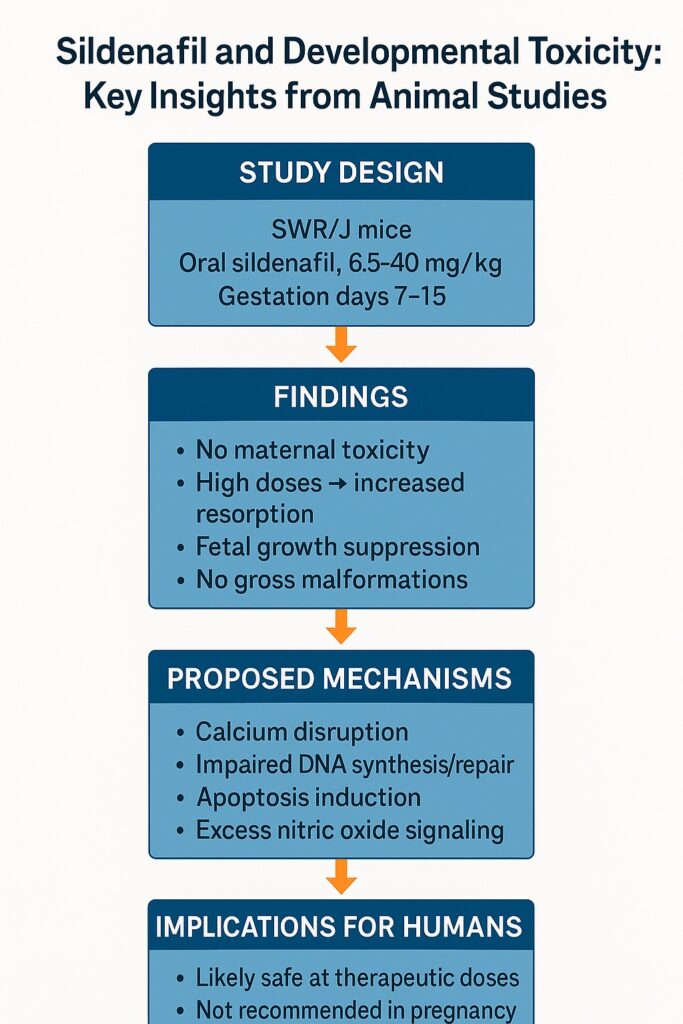
Animal studies serve as the cornerstone for investigating potential teratogenic and developmental effects of drugs. The study under discussion used inbred SWR/J mice, a strain often employed in toxicological and developmental research. These mice were selected for their genetic uniformity and predictable reproductive characteristics, which minimize variability in experimental outcomes.
Pregnant mice were exposed to graded doses of sildenafil citrate ranging from 6.5 to 40.0 mg/kg body weight, administered orally. These dosages were carefully chosen to represent multiples of the standard 50 mg human dose, thus allowing translation into clinically relevant scales. Importantly, the drug was given during specific gestational windows: days 7–9, 10–12, and 13–15 of pregnancy, which correspond to critical periods of organogenesis and fetal growth in mice.
The study assessed multiple endpoints:
- Maternal mortality and overt toxicity signs.
- Rates of resorption (a marker of embryonic loss).
- Numbers of live and dead fetuses per litter.
- Fetal body weight as an indicator of intrauterine growth.
- External, internal, and skeletal malformations detected by detailed microscopic and staining techniques.
By applying these rigorous methodologies, the investigators could discern not only whether sildenafil induced gross malformations but also whether subtler effects such as growth suppression or embryonic loss occurred.
Maternal Safety and Observed Tolerance
One of the first reassuring findings was the absence of maternal mortality or overt toxicity at all tested dose levels. Pregnant females tolerated sildenafil without visible clinical distress, changes in behavior, or systemic illness. This result underscores that, at least in adult organisms, sildenafil does not exert acute toxic effects even when administered at doses significantly higher than those used therapeutically in humans.
However, maternal well-being does not guarantee embryonic safety. Drugs can bypass maternal systems and exert specific effects on developing embryos, where cellular processes are highly sensitive to external perturbations. Thus, the lack of maternal toxicity provided a baseline but did not exclude the possibility of adverse outcomes in the fetuses.
Fetal Outcomes: Growth Suppression and Embryo-Fetotoxicity
The results for fetal development painted a more nuanced picture. At lower and intermediate doses (6.5–19.5 mg/kg), sildenafil exposure did not produce significant abnormalities. Fetuses appeared normal, with no evidence of skeletal malformations, internal defects, or external anomalies. This aligns with previous reports suggesting that sildenafil is not a classical teratogen.
However, the picture changed at higher doses (26.0, 32.5, and 40.0 mg/kg). Several key findings emerged:
- Resorption rates increased significantly, particularly when sildenafil was given during days 13–15 of gestation. This suggests embryo-fetal loss at later stages of development.
- Fetal body weight declined in a dose-dependent manner, with the 40.0 mg/kg group consistently showing suppressed growth across all gestational windows.
- No gross malformations were observed, even at the highest doses, indicating that sildenafil does not directly induce structural teratogenesis.
These findings reveal that sildenafil’s primary developmental effects are functional rather than morphological: it suppresses fetal growth and increases embryo-fetal mortality but does not cause overt malformations. Such distinctions are important when extrapolating risks to human pregnancies, where growth restriction and miscarriage represent clinically significant but mechanistically different outcomes than structural birth defects.
Mechanistic Insights: Why Might Sildenafil Affect Embryos?
Understanding why sildenafil exerts these effects requires delving into cellular and molecular mechanisms. Several plausible pathways have been proposed:
- Altered Calcium Dynamics
Intracellular calcium plays a central role in embryonic cell division and differentiation. PDE inhibitors, by modifying cyclic nucleotide pathways, may disrupt calcium flux and distribution. Abnormal calcium signaling has been shown to impair normal embryogenesis, and sildenafil’s elevation of cGMP may indirectly disturb this delicate balance. - Effects on DNA Synthesis and Repair
Other PDE inhibitors, such as pentoxifylline, have been shown to impair DNA synthesis and repair mechanisms. Similar actions could occur with sildenafil, leading to slowed proliferation of embryonic cells and consequently reduced fetal growth. - Induction of Apoptosis
Sustained elevation of cGMP can trigger apoptotic pathways in certain cell types. If sildenafil prolongs high cGMP levels in embryonic tissues, this could result in programmed cell death, contributing to resorption or growth suppression. - Nitric Oxide Overproduction
Sildenafil enhances nitric oxide (NO)-mediated signaling. While NO is essential for vascular regulation, excessive NO levels in embryonic tissues can be cytotoxic and may impair implantation or reduce fetal survival.
Together, these mechanisms highlight that sildenafil’s impact on embryonic development is likely multifactorial, involving disruptions of normal signaling cascades crucial for growth and survival.
Clinical Implications and Human Relevance
The translational significance of these animal findings must be interpreted cautiously. Several points merit discussion:
- Therapeutic Doses vs. Experimental Doses
The highest dose used in mice (40 mg/kg) represents a multiple of human therapeutic exposure. Nevertheless, dose extrapolation between species is not linear. While the findings raise concerns, they may not directly predict effects in women using standard sildenafil doses for approved indications. - Emerging Uses in Obstetrics
Sildenafil has been explored as a potential therapy for intrauterine growth restriction (IUGR) and preterm labor, based on its ability to enhance uteroplacental blood flow. Ironically, while the intention is to promote fetal growth, animal evidence suggests that excessive exposure could produce the opposite outcome. This paradox underscores the need for carefully designed clinical trials and cautious interpretation of animal data. - Male Use and Reproductive Risks
Since sildenafil is widely used by men of reproductive age, questions arise about indirect effects on offspring via paternal exposure. Some in vitro studies suggest sildenafil may alter sperm function, though the clinical relevance remains debated. The current mouse study focused on maternal exposure, but the broader reproductive safety profile should consider both parental contributions. - Counseling Pregnant Women
Although sildenafil is not prescribed to pregnant women for erectile dysfunction, accidental or off-label use may occur. The available evidence indicates that while gross malformations are unlikely, risks of growth suppression or fetal loss cannot be dismissed. Clinicians should therefore exercise caution and avoid unnecessary exposure during pregnancy.
Limitations of the Study
Like all animal research, the SWR/J mouse study carries limitations:
- Species Differences
Mouse physiology does not perfectly mirror human pregnancy, and drug metabolism differs significantly. - Restricted Gestational Windows
The study focused on specific gestational periods (days 7–15), which may not capture potential effects at later stages of pregnancy. - Lack of Long-Term Outcomes
Only immediate fetal outcomes were assessed; postnatal survival, growth, and reproductive performance were not studied.
Despite these caveats, the results provide valuable early warnings and justify further research in more advanced models and carefully controlled human trials.
Conclusion
The study of sildenafil citrate in SWR/J mice provides a critical reminder that drugs designed for adult indications may exert unexpected effects during development. While sildenafil did not induce malformations, its growth-suppressing and embryo-fetotoxic effects at higher doses highlight the sensitivity of embryonic systems to pharmacological perturbations.
For clinicians, the take-home message is one of balance: sildenafil remains safe and effective in its approved indications when used responsibly, but its role in pregnancy or reproductive medicine must be approached with caution. Further research will determine whether the benefits of sildenafil in conditions like IUGR outweigh the potential risks suggested by animal data. Until then, prudence, informed counseling, and rigorous evidence-based practice remain the guiding principles.
FAQ
1. Does sildenafil cause birth defects in humans?
Current evidence, including animal studies, indicates that sildenafil does not cause structural malformations. However, high doses may suppress fetal growth or increase embryonic loss. Human data remain limited, so sildenafil is not routinely recommended during pregnancy.
2. Why has sildenafil been studied for use in pregnancy if it may be toxic?
Sildenafil improves blood flow by dilating blood vessels, which led researchers to test it for intrauterine growth restriction (IUGR) and preterm labor. The hope was to improve placental perfusion and fetal growth. Unfortunately, some trials and animal studies suggest potential risks, highlighting the need for caution.
3. Should men taking sildenafil worry about effects on future children?
Evidence suggests that standard sildenafil use by men is unlikely to harm future offspring. While some laboratory studies show effects on sperm function, these do not translate clearly into real-world risks. Still, long-term studies are limited, and moderation in use remains advisable.