Introduction
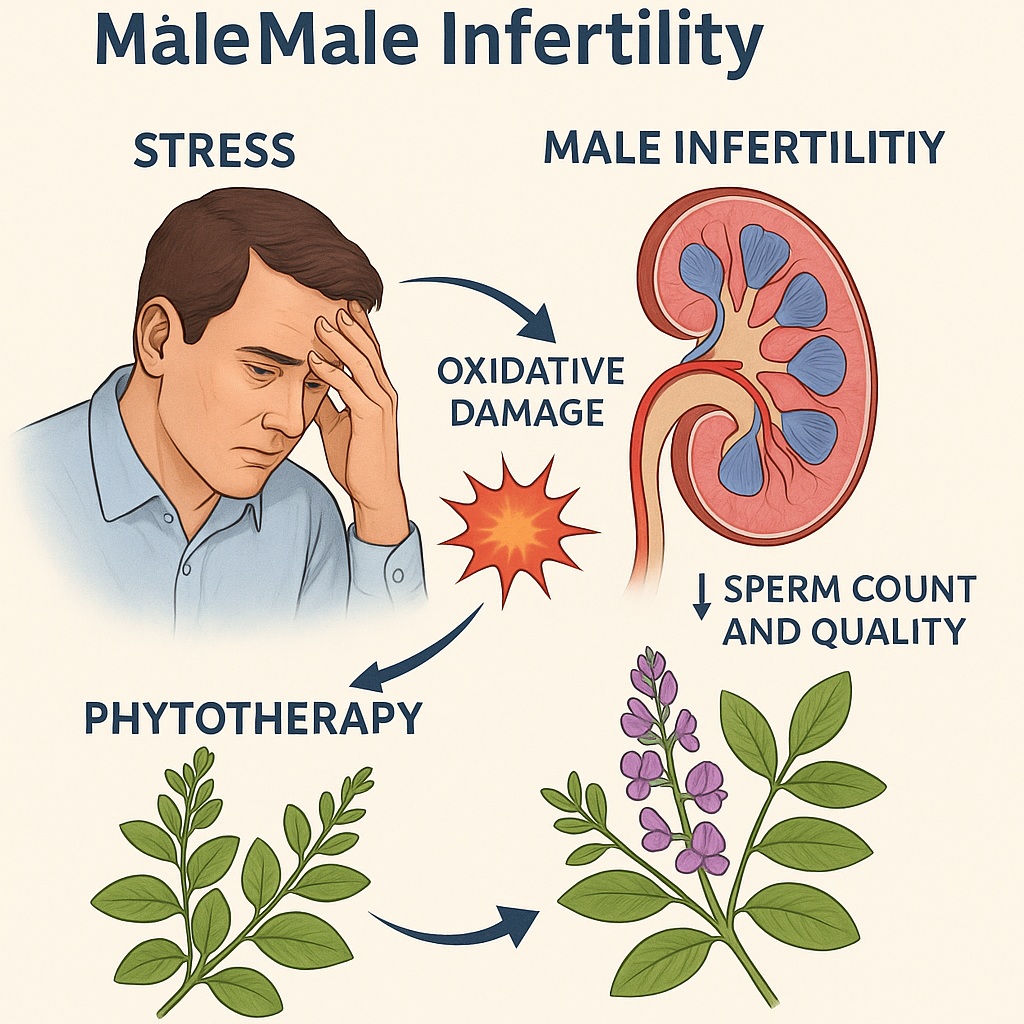
Infertility is no longer a whispered diagnosis confined to the shadows of medical practice. It has become a prominent global health concern, affecting approximately 15% of couples worldwide. Among the causes, male infertility contributes nearly 50%, underscoring the critical importance of understanding male reproductive health. While infectious diseases, congenital abnormalities, and genetic disorders play their roles, two modern enemies stand out: psychological stress and oxidative stress. These twin burdens, fueled by modern lifestyles, significantly impair spermatogenesis and compromise sperm quality, with devastating effects on male fertility.
Conventional therapies, such as hormonal treatments, antioxidants, and assisted reproductive technologies, have yielded mixed outcomes. Their efficacy is often limited, side effects are not trivial, and access remains uneven, especially in resource-limited settings. This has fueled interest in plant-based medicines and natural products, which have long histories in traditional healing systems. Among them, Desmodium gangeticum (DG), a leguminous plant used in Ayurveda, has emerged as a potential ally in restoring reproductive balance. Traditionally valued for its antioxidant, adaptogenic, and anti-inflammatory properties, DG has gained renewed attention in modern pharmacological research.
The recent study published in 2024 explored DG’s influence on male infertility using a multi-modal framework. By combining in vivo animal models, in vitro biochemical assays, and in silico computational analyses, the researchers mapped DG’s therapeutic potential with remarkable precision. Their work provides an integrated view of how phytoconstituents might counteract the deleterious effects of stress on the male reproductive axis. The story of DG, therefore, is not simply one of herbal folklore, but one of careful scientific validation that bridges ancient wisdom with modern pharmacology.
Stress, Oxidative Damage, and Male Infertility
To understand DG’s therapeutic promise, one must first grasp how stress disrupts male fertility. Psychological stress activates the hypothalamic–pituitary–adrenal (HPA) axis, releasing glucocorticoids such as cortisol. While adaptive in acute settings, chronic elevation suppresses the hypothalamic–pituitary–gonadal (HPG) axis, leading to reduced gonadotropin secretion and impaired testosterone synthesis. The result is a hormonal milieu hostile to spermatogenesis.
Oxidative stress adds further insult. Spermatozoa, with their rich polyunsaturated fatty acid membranes and limited antioxidant defenses, are exquisitely vulnerable to reactive oxygen species (ROS). Excess ROS damages DNA, fragments mitochondrial membranes, and compromises motility. Markers such as malondialdehyde (MDA) rise, reflecting lipid peroxidation, while antioxidant enzymes like superoxide dismutase (SOD) and catalase (CAT) decline.
The dual burden of psychological and oxidative stress therefore establishes a vicious cycle: impaired spermatogenesis lowers sperm count and quality, while reduced fertility feeds psychological distress, perpetuating further HPA axis activation. Breaking this cycle requires agents that are simultaneously antioxidant, adaptogenic, and hormone-modulating—a trifecta that DG appears well-suited to deliver.
Desmodium gangeticum: Ethnopharmacology Meets Modern Science
Desmodium gangeticum, commonly known as Salparni in Ayurveda, has been used for centuries to treat fevers, inflammatory conditions, and nervous disorders. Its roots and aerial parts are rich in alkaloids, flavonoids, isoflavones, and phenolic compounds, many of which display antioxidant and adaptogenic activities. Traditional healers employed DG in disorders of the nervous system and reproductive weakness, suggesting a longstanding belief in its restorative properties.
Modern phytochemical analyses confirm that DG contains compounds such as gangetin, desmodin, and various flavonoid glycosides. These constituents have been shown to scavenge free radicals, reduce lipid peroxidation, and modulate inflammatory cascades. Some also appear to influence neurotransmitter systems, aligning with the concept of adaptogenic activity.
Yet, traditional knowledge alone cannot guide clinical translation. The present multi-modal study advanced beyond descriptive ethnobotany by testing DG in stress-induced infertility models, thereby providing mechanistic insights that connect phytoconstituents with biological targets.
In Vivo Findings: Animal Models of Stress-Induced Infertility
The in vivo arm of the study used rodent models subjected to chronic stress protocols, such as restraint stress, to mimic the psychological and physiological strain experienced in humans. Animals exposed to stress displayed hallmark features of infertility: reduced testicular weight, diminished sperm count and motility, elevated oxidative stress markers, and disrupted hormone levels.
When treated with DG extract, these deleterious changes were markedly reversed. Testicular histopathology revealed restored seminiferous tubule architecture, with increased germ cell proliferation and normalized Leydig cell function. Sperm parameters improved significantly: both count and motility rose toward baseline levels, and morphological abnormalities declined.
On the biochemical front, DG supplementation reduced MDA levels, reflecting diminished lipid peroxidation, and restored antioxidant enzyme activity (SOD, CAT, and glutathione peroxidase). Serum testosterone levels rebounded, suggesting that DG not only mitigated oxidative damage but also revived the steroidogenic machinery of the testes. These improvements collectively positioned DG as a robust protector against stress-induced infertility in vivo.
In Vitro Mechanistic Insights
The in vitro experiments probed DG’s antioxidant and enzyme-modulating properties under controlled conditions. Extracts of DG demonstrated potent free radical scavenging activity in DPPH and nitric oxide assays. They also inhibited lipid peroxidation in membrane models, corroborating the in vivo findings of reduced oxidative stress.
Crucially, DG was found to influence the activity of key reproductive enzymes. Aromatase and 5-alpha-reductase, enzymes central to androgen metabolism, showed modulation in the presence of DG phytochemicals. By maintaining balanced androgenic signaling, DG may preserve testosterone bioavailability and prevent estrogen dominance, both of which are critical for spermatogenesis.
These in vitro insights strengthen the hypothesis that DG’s fertility-enhancing effects are not solely due to generic antioxidant activity. Instead, they reflect a targeted modulation of reproductive biochemistry, integrating endocrine, enzymatic, and redox homeostasis.
In Silico Findings: Docking and Molecular Modeling
The in silico component of the study used computational docking to predict interactions between DG phytoconstituents and protein targets implicated in stress and reproduction. Docking analyses suggested strong binding affinities between DG flavonoids and enzymes such as aromatase, 17β-hydroxysteroid dehydrogenase, and heat shock proteins.
Heat shock proteins, which are upregulated during cellular stress, play dual roles in protecting cells but also in amplifying stress responses when dysregulated. DG phytochemicals demonstrated potential to stabilize these proteins, thereby reducing stress-induced apoptosis in germ cells.
Moreover, molecular dynamics simulations suggested stable ligand–protein interactions, lending credibility to the docking predictions. These computational findings not only validate the in vivo and in vitro results but also provide a map for drug discovery, highlighting specific DG molecules as templates for novel infertility therapeutics.
Integrative Mechanisms: A Systems-Level Perspective
When viewed together, the multi-modal results converge on a systems-level mechanism:
- DG reduces oxidative stress, sparing spermatozoa from ROS-mediated damage.
- DG restores hormonal balance by supporting testosterone biosynthesis and modulating androgenic enzymes.
- DG enhances testicular architecture, ensuring proper spermatogenesis.
- DG phytochemicals interact with stress-response proteins, improving cellular resilience.
This integrated action profile makes DG an unusually promising candidate. Unlike single-target drugs, which may falter against the multifactorial nature of infertility, DG provides a multi-pronged defense that mirrors the complexity of the disease.
Such systems pharmacology is precisely what modern medicine requires: agents capable of harmonizing multiple dysregulated pathways without overwhelming toxicity.
Clinical Translation: From Bench to Bedside?
While the preclinical findings are compelling, clinical translation remains a challenge. Several considerations emerge.
First, the standardization of extracts is paramount. Herbal preparations vary widely depending on geography, cultivation, and processing. Without standardized phytochemical profiles, reproducibility across studies and clinics will be elusive.
Second, dose optimization and safety profiling must be addressed. Though DG has been used traditionally with apparent safety, formal toxicological evaluations are necessary. Long-term use in reproductive-age men requires careful monitoring of hormonal balance, liver function, and cardiovascular parameters.
Third, controlled clinical trials will be essential to validate efficacy in humans. The psychological and oxidative stress burdens in human infertility are complex, and while rodent models are informative, human physiology may reveal additional challenges or opportunities.
Nevertheless, the present study lays a solid foundation for such trials, offering both mechanistic rationale and proof-of-concept data that DG can restore fertility parameters under stress conditions.
Broader Implications: Stress, Fertility, and Phytomedicine
The story of DG is part of a broader narrative in reproductive medicine: the increasing recognition that infertility is not merely a disorder of gametes, but a systemic condition shaped by the interplay of stress, oxidative biology, and endocrine regulation. Addressing infertility therefore requires strategies that move beyond narrowly defined drug targets.
Phytomedicine, with its rich array of bioactive compounds, is uniquely suited to this challenge. Unlike synthetic drugs designed for single targets, plants like DG contain complex phytochemical “cocktails” that engage multiple pathways simultaneously. This polypharmacology is sometimes viewed as a liability in conventional drug development, but in multifactorial disorders like infertility, it may be precisely the strength needed.
Furthermore, the use of in silico and systems-biology tools ensures that modern phytomedicine does not retreat into anecdotal empiricism. Instead, it moves forward with the same scientific rigor applied to synthetic drug discovery, integrating tradition with technology.
Conclusion
The 2024 multi-modal investigation of Desmodium gangeticum underscores the plant’s potential as a therapeutic agent against stress-induced male infertility. By combining in vivo, in vitro, and in silico approaches, the study mapped a detailed mechanistic landscape: DG protects sperm and testes from oxidative stress, restores hormonal balance, and stabilizes stress-response proteins. These converging actions highlight DG’s promise as a holistic intervention for reproductive dysfunction.
Translation to clinical practice will require standardized formulations, rigorous safety testing, and well-designed clinical trials. Yet, the trajectory is clear: DG exemplifies how ancient remedies can gain new life in modern medicine when evaluated with contemporary scientific tools.
Ultimately, DG’s story reminds us that infertility, though complex, may be countered not only with high-tech interventions but also with carefully harnessed natural products that work in harmony with human physiology. The blending of ethnopharmacology and modern science offers a pathway of hope for millions of couples worldwide facing the burden of infertility.
FAQ
1. Can Desmodium gangeticum be recommended for men with infertility today?
Not yet. While preclinical studies are promising, clinical trials in humans are needed to confirm efficacy and safety. At present, DG should be viewed as an experimental candidate, not a standard therapy.
2. How does DG differ from standard antioxidant supplements for infertility?
Unlike single antioxidants such as vitamin C or E, DG contains multiple phytochemicals that act on oxidative stress, hormonal regulation, and stress-response proteins simultaneously. This multi-targeted profile may provide broader protection.
3. Are there risks associated with DG use?
Traditional use suggests safety, but formal toxicology studies are limited. Potential risks include hormonal imbalance or liver enzyme modulation at high doses. Until clinical safety data are available, DG should not be self-administered for infertility.