Introduction: When a Paralyzing Toxin Turns Therapeutic
The history of medicine is full of paradoxes, but few are as striking as the story of Clostridium botulinum—the bacterium that produces the world’s most potent toxin—and its transformation into one of the most versatile therapeutic agents. Initially feared for its lethal paralysis, botulinum neurotoxin type A (BoNT-A) has become a cornerstone in the management of neurological, urological, and even aesthetic disorders.
Now, researchers are exploring yet another frontier: the potential role of BoNT-A in the treatment of erectile dysfunction (ED). The idea is as provocative as it is promising. Could the same compound that erases wrinkles and relaxes dystonic muscles also relax the cavernosal smooth muscle of the penis, restoring erectile function where traditional therapies fail?
Recent pilot studies suggest that this audacious concept might have merit. Human and animal research conducted in Egypt and Canada has demonstrated that intracavernosal injection of BoNT-A can improve penile hemodynamics, enhance erection hardness, and even convert pharmacologic non-responders into responders. Yet, as with any innovation in sexual medicine, the path from bench to bedside demands rigorous scientific scrutiny.
Understanding the Toxin: Mechanism of Action Beyond Cosmetics
BoNT-A exerts its effects by blocking acetylcholine release at cholinergic synapses, leading to temporary flaccid paralysis of targeted muscles. This mechanism is mediated through interference with the SNARE complex, specifically the synaptosome-associated protein 25 (SNAP-25), a key mediator of synaptic vesicle fusion and neurotransmitter release.
Structurally, BoNT-A consists of a heavy and light chain connected by a disulfide bond. The heavy chain binds with high specificity to cholinergic neurons, allowing the toxin to enter via endocytosis. Once internalized, the light chain cleaves SNAP-25, effectively silencing the synaptic terminal. Recovery requires the formation of new nerve terminals—a process that typically takes 2–3 months in striated muscle, but may persist longer in smooth muscle.
Interestingly, BoNT-A’s effects are not limited to acetylcholine. Evidence indicates inhibition of noradrenaline, dopamine, glycine, and GABA, depending on dose and injection site. This broader neuromodulatory capacity underpins its therapeutic diversity—from ocular muscle relaxation to the suppression of bladder overactivity.
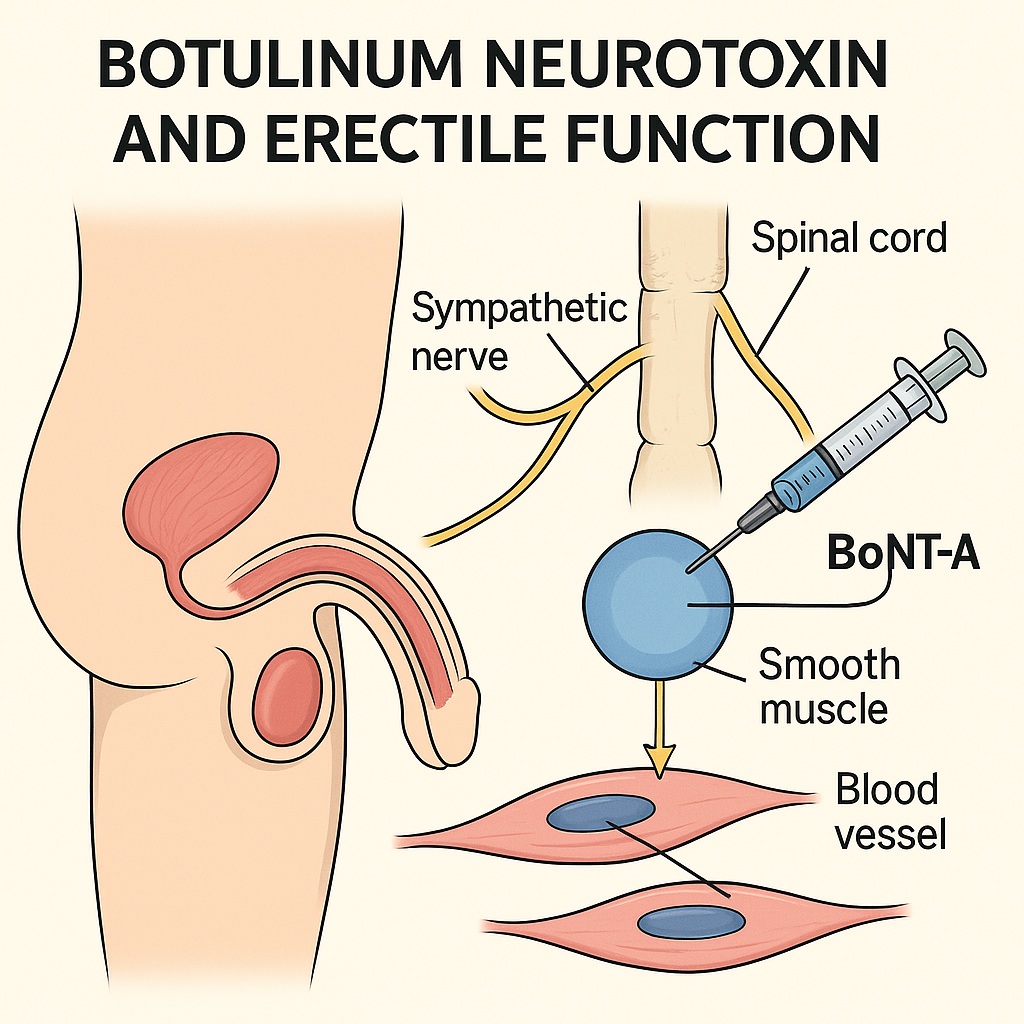
The leap from the bladder to the penis is, physiologically speaking, not that far. Both involve autonomic control of smooth muscle tone, and both can suffer from excessive contractility or dysregulated neural input. This conceptual bridge has opened the way for experimental use of BoNT-A in corpora cavernosa, where its capacity to reduce sympathetic tone may help overcome one of the central pathophysiologic obstacles in vasculogenic ED.
Erectile Physiology Revisited: The Balance Between Contraction and Relaxation
A penile erection is a marvel of integrated neurovascular physiology. It requires a precisely timed shift from sympathetic dominance (contraction) to parasympathetic activation (relaxation) within the corpus cavernosum.
In the flaccid state, tonic sympathetic discharge maintains cavernosal smooth muscle contraction, restricting blood inflow. Upon sexual stimulation, non-adrenergic, non-cholinergic (NANC) neurons release nitric oxide (NO), activating cyclic guanosine monophosphate (cGMP) signaling pathways that promote smooth muscle relaxation. As cavernosal sinusoids expand, venous outflow is compressed against the tunica albuginea, resulting in rigidity.
Detumescence occurs when phosphodiesterase type 5 (PDE5) degrades cGMP, restoring smooth muscle tone and vascular resistance.
This balance is easily disrupted. Aging, diabetes, hypertension, and hyperlipidemia all impair endothelial NO production and increase sympathetic vasoconstriction, leading to the hallmark feature of vasculogenic ED—insufficient cavernosal relaxation. When oral PDE5 inhibitors fail, the issue often lies not in cGMP metabolism, but in overactive sympathetic tone or fibrotic changes within the cavernosal tissue itself.
Herein lies the rationale for BoNT-A: by silencing sympathetic neurotransmission and relaxing smooth muscle, it could theoretically restore cavernosal compliance and improve responsiveness to existing therapies.
Current Medical Uses of BoNT-A: A Legacy of Controlled Relaxation
BoNT-A’s clinical journey began in 1977, when ophthalmologists used it to treat strabismus in children. Since then, it has gained approval for an impressive list of conditions:
- Neurologic disorders: dystonias, spasticity, tremor, hemifacial spasm.
- Autonomic dysfunctions: hyperhidrosis, hypersalivation, and chronic migraine.
- Smooth muscle disorders: achalasia, esophageal spasm, and overactive bladder.
In urology, BoNT-A has revolutionized the management of neurogenic detrusor overactivity and idiopathic overactive bladder. Large phase III trials, such as EMBARK and DIGNITY, demonstrated significant improvements in bladder capacity, detrusor pressure, and quality of life, with minimal systemic effects.
These data provided a critical foundation of safety and feasibility for local injections in smooth muscle tissues—including, potentially, the penis. The predictable duration of action (3–6 months) and reversible nature of its effects make BoNT-A an appealing candidate for conditions requiring transient functional modulation.
A New Hypothesis Emerges: BoNT-A in Erectile Dysfunction
The concept of injecting BoNT-A directly into the corpora cavernosa originated from clinical serendipity. Researchers in Egypt with experience in both anti-aging cosmetic BoNT-A applications and andrology observed that the toxin’s muscle-relaxing effect might logically extend to penile tissue.
The rationale was elegantly simple: since cavernosal smooth muscle relaxation is the central mechanism of erection, and BoNT-A induces smooth muscle relaxation by inhibiting neurotransmitter release, then intracavernosal BoNT-A might benefit men with refractory vasculogenic ED—especially those who failed to respond to PDE5 inhibitors or intracavernosal pharmacotherapy.
In 2015, this idea evolved from hypothesis to reality with the first pilot human trial, followed by animal studies that provided histologic validation.
Evidence from Clinical and Experimental Studies
Human Trials
Two human studies conducted in Cairo form the cornerstone of current evidence. The first, a phase I randomized controlled pilot trial, enrolled 24 men with severe vasculogenic ED unresponsive to PDE5 inhibitors and intracavernosal therapy. Participants were randomized to receive 50 units of BoNT-A or saline via intracavernosal injection.
Key findings included:
- Peak systolic velocity (on penile Doppler) increased from 24.6 to 34.9 cm/s in the BoNT-A group (p = 0.005).
- Erection Hardness Score (EHS) improved from 2.0 to 2.75 (p = 0.01).
- Sexual Health Inventory for Men (SHIM) scores rose from 5.6 to 10.3 (p = 0.007).
- Seven of twelve treated men achieved penetrative intercourse with sildenafil assistance, compared to only two in the control group.
Adverse events were minimal—only one patient experienced a transient prolonged erection (2.5 hours) that resolved with intracavernosal ephedrine.
A phase II trial involving 160 men (100-unit dose) is currently underway, aiming to determine optimal dosage and duration of effect.
Animal Studies
Parallel experiments in rat models provided mechanistic support. In these studies, intracavernosal BoNT-A led to a significant increase in resting sinusoidal diameter and intracavernosal pressure during nerve stimulation, confirming direct smooth muscle relaxation. Histologic sections revealed expanded cavernosal spaces without evidence of tissue necrosis or systemic toxicity.
Interestingly, the effective doses in rats were proportionally higher (1–2 units), corresponding to approximately 100–200 units in humans, yet no priapism or neurotoxicity occurred.
Together, these results form a coherent narrative: BoNT-A, when locally administered, can reduce cavernosal tone, enhance perfusion, and restore erectile responsiveness in previously refractory cases.
How Does It Work? A Neurovascular Perspective
The proposed mechanism of BoNT-A in erectile enhancement centers on modulation of autonomic neurotransmission within penile tissue.
In the normal state, sympathetic fibers release noradrenaline, which binds to α-adrenergic receptors on smooth muscle, promoting contraction and detumescence. By inhibiting noradrenaline release, BoNT-A effectively removes this baseline constrictive tone, allowing the parasympathetic (NO-mediated) pathway to dominate.
Simultaneously, BoNT-A may also inhibit cholinergic transmission, but this effect is outweighed by its net facilitation of relaxation. The end result is a physiological environment favoring easier penile engorgement in response to stimulation or pharmacologic agents.
In simple terms: BoNT-A doesn’t “cause” erections—it removes the brakes that prevent them. The restored cavernosal compliance allows PDE5 inhibitors or endogenous arousal signals to work more effectively, converting non-responders into responders.
Safety and Practical Considerations
The notion of injecting a neurotoxin into the penis naturally invites caution. However, the available data are reassuring.
In both human and animal studies, no systemic toxicity was observed. The localized dose (50–100 units) is substantially lower than those used in detrusor muscle injections for bladder overactivity. Moreover, BoNT-A has a high molecular weight and limited systemic diffusion, particularly when the penile base is compressed after injection—a simple maneuver that reduces vascular uptake.
The primary concern, priapism, was not observed beyond one mild case, suggesting that complete smooth muscle paralysis does not occur. The relaxation induced is partial and reversible, restoring function without destabilizing the erection reflex arc.
In practical terms, if future studies confirm efficacy, intracavernosal BoNT-A could be administered in an outpatient setting, much like current ICI therapy, with effects lasting 3–6 months and repeatable dosing thereafter.
Challenges and Questions Ahead
While the concept is scientifically sound, several key questions remain unanswered:
- Optimal dosing: Early trials used 50–100 units, but dose-response relationships are uncharted.
- Duration of efficacy: Smooth muscle recovery time may differ across individuals.
- Long-term safety: Repeated injections could potentially alter local neuromuscular architecture.
- Patient selection: BoNT-A may be most effective in vasculogenic rather than neurogenic ED, but larger comparative studies are needed.
Moreover, while early data suggest synergy with sildenafil, it remains unclear whether BoNT-A alone can sustain functional erections or whether it primarily serves as an adjunctive therapy.
Beyond the Penis: A Broader Therapeutic Implication
The exploration of BoNT-A in erectile function is part of a larger trend—using neurotoxins to recalibrate autonomic balance in urology. Its success in treating detrusor overactivity, chronic prostatitis, and pelvic floor spasm underscores a paradigm shift: functional genitourinary disorders are not solely structural or hormonal—they are neurochemical.
In this context, the penis can be viewed as another target organ where neural hyperactivity or sympathetic excess contributes to dysfunction. Modulating that activity pharmacologically could represent a new class of neuromodulatory treatments—less invasive than implants, more specific than systemic drugs.
BoNT-A might thus occupy a unique niche: a bridge between pharmacotherapy and surgery for men at the end of the medical treatment spectrum.
The Future of Research: From Pilot Trials to Precision Andrology
The road ahead requires multicenter, double-blinded, randomized controlled trials with extended follow-up periods and standardized protocols. Histologic studies should explore nerve fiber regeneration, smooth muscle remodeling, and molecular changes in SNARE protein expression.
Equally important is defining biomarkers of response—for example, baseline sympathetic tone, cavernosal compliance, or endothelial nitric oxide synthase (eNOS) activity—that could predict which patients will benefit most.
If validated, BoNT-A could complement or even reduce the need for penile prosthesis implantation, offering a minimally invasive option for otherwise hopeless cases.
Conclusion: From Paralysis to Potency
Few therapeutic journeys better capture the creativity of modern medicine than that of botulinum toxin. What began as a deadly poison now stands as a potential ally in restoring one of humanity’s most fundamental physiological functions.
BoNT-A’s emerging role in erectile dysfunction is not a reinvention of the wheel—it is an evolution in thinking. By targeting the neural regulation of penile smooth muscle, it addresses a gap left by current treatments. The early data, though preliminary, suggest real potential: improved hemodynamics, restored pharmacologic responsiveness, and minimal risk.
If future trials confirm safety and efficacy, BoNT-A may soon redefine the therapeutic hierarchy in ED management—an elegant example of how neurobiology and sexual medicine can converge to turn paralysis into potency.
FAQ: Botulinum Neurotoxin and Erectile Dysfunction
1. How does BoNT-A differ from traditional ED treatments like sildenafil?
Sildenafil enhances nitric oxide signaling to promote vasodilation, but it requires functional cavernosal relaxation. BoNT-A, by contrast, reduces sympathetic tone, making the cavernosal tissue more receptive to nitric oxide. It addresses the upstream neural control rather than the downstream vascular response.
2. Is intracavernosal BoNT-A safe?
Preliminary studies show that localized injections of 50–100 units are well tolerated, with no systemic toxicity and only rare transient prolonged erections. However, long-term safety data from larger trials are still pending.
3. Who might benefit most from BoNT-A therapy?
BoNT-A is particularly promising for men with vasculogenic ED who have failed PDE5 inhibitors and standard injection therapies, but who are not ready or suitable for penile prosthesis surgery.