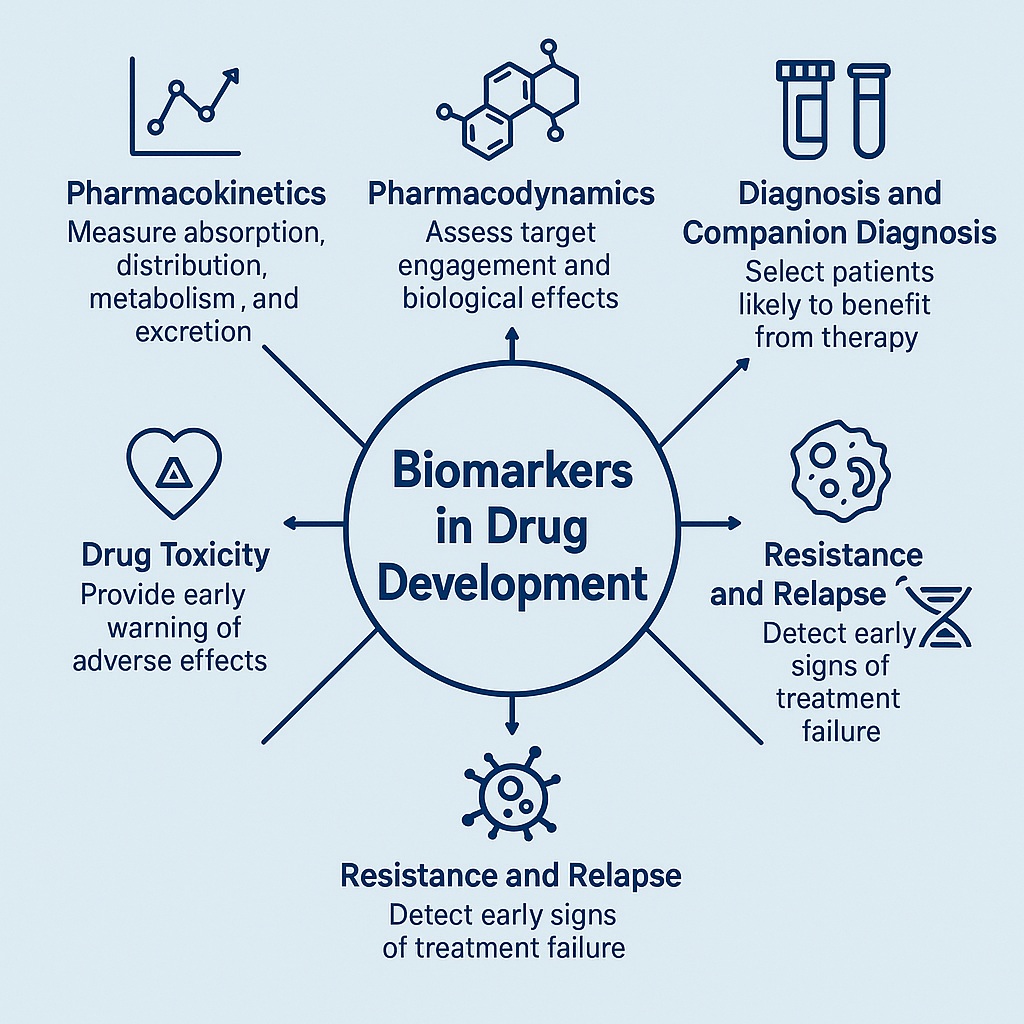
Introduction
The science of medicine has always been a dialogue between observation and measurement. For centuries, physicians relied on symptoms, physical signs, and intuition to guide therapy. But the past few decades have ushered in a profound transformation—one that leans heavily on biomarkers. These measurable indicators of biological processes, whether normal or pathological, now serve as the compass guiding drug development, from discovery to post-market surveillance.
Biomarkers are no longer mere laboratory curiosities. They have become fundamental in the architecture of modern medicine, shaping clinical trial design, personalizing treatments, accelerating regulatory approval, and even predicting adverse events. Their use represents the very essence of precision medicine: tailoring the right drug, at the right dose, to the right patient, at the right time.
This article unpacks the evolving role of biomarkers in pharmacology, therapeutic effectiveness, and drug safety. We will explore how they redefine pharmacokinetics, pharmacodynamics, diagnosis, prediction, resistance, and toxicity monitoring—culminating in a critical discussion of future perspectives.
Pharmacokinetics: Following the Journey of a Drug
Pharmacokinetics (PK) describes what the body does to a drug. From absorption and distribution to metabolism and excretion, PK determines whether a therapy achieves its therapeutic goal—or fails spectacularly. Biomarkers serve as quantifiable checkpoints along this journey.
Take sildenafil, a widely known drug for erectile dysfunction. In bioequivalence studies, plasma levels of sildenafil and its metabolite N-desmethyl-sildenafil act as PK biomarkers, ensuring that generic versions match the efficacy of the original. Such measurements save time and cost compared with full-scale clinical trials.
Another elegant example comes from biologics. Antibody–drug conjugates (ADCs) like trastuzumab emtansine are complex hybrids of antibodies and cytotoxic payloads. Measuring circulating concentrations of the conjugate, antibody, and free payload provides insights into how effectively the drug reaches its target while avoiding systemic toxicity. Without biomarkers, tracking such multi-layered pharmacology would be near impossible.
Even endogenous proteins like serum albumin can act as PK biomarkers. Liraglutide, a GLP-1 analogue for diabetes, achieves prolonged half-life by reversible albumin binding. In patients with severe liver disease, reduced albumin alters drug levels—an important reminder that biomarkers are sometimes hiding in plain sight.
Pharmacodynamics: What the Drug Does to the Body
If PK is the voyage of the drug, pharmacodynamics (PD) is its effect on the destination. Here, biomarkers function as litmus tests for whether a drug engages its intended target and exerts the expected biological effect.
In early-phase trials, PD biomarkers often decide a drug’s fate. For instance, veliparib, a PARP inhibitor, was evaluated in a phase 0 trial—where suppression of PARP activity in tumor tissue confirmed that the drug hit its target. Without this biomarker readout, further trials would have been speculative at best.
Diabetes management offers another illustration. HbA1c has long been the gold-standard PD biomarker, reflecting average glucose control over months. Newer antidiabetic drugs are benchmarked against changes in HbA1c, but researchers also correlate it with cardiovascular and renal biomarkers to capture long-term outcomes.
In infectious disease, procalcitonin (PCT) serves as a PD biomarker guiding antibiotic therapy. Declining PCT levels prompt physicians to de-escalate antibiotics, reducing risks of resistance and toxicity. Here, biomarkers not only validate efficacy but actively shape therapeutic strategy.
Diagnosis and Monitoring: Biomarkers as Clinical Gatekeepers
Drug development cannot proceed without clear diagnostic criteria to identify the right patient population. Biomarkers, in this context, become gatekeepers of eligibility and monitoring tools for treatment response.
Classic examples include prostate-specific antigen (PSA) for prostate cancer and alpha-fetoprotein for hepatocellular carcinoma. These markers have long underpinned clinical decision-making. But modern advances bring more sophisticated counterparts.
During the COVID-19 pandemic, PCR-based viral RNA detection became the diagnostic biomarker par excellence, guiding patient isolation, therapy, and vaccine evaluation. In oncology, BCR-ABL fusion proteins serve as both diagnostic and therapeutic biomarkers for chronic myeloid leukemia.
Perhaps the most striking evolution is seen in hemophilia. For decades, factor VIII and IX levels defined diagnosis and therapy. Today, gene therapies and bispecific antibodies like emicizumab are designed explicitly to restore or mimic these biomarker-defined deficiencies. In other words, the biomarker itself has become the therapeutic target.
Prediction and Companion Diagnosis: The Dawn of Precision Medicine
Not all patients with the same disease respond to treatment equally. Predictive biomarkers, often developed alongside companion diagnostics, allow clinicians to pre-select those most likely to benefit.
The oncology field exemplifies this shift. Trastuzumab for HER2-positive breast cancer, alectinib for ALK-rearranged lung cancer, and pembrolizumab for MSI-high colorectal cancer—each is inseparable from its predictive biomarker. Without the biomarker, these drugs would be statistical failures; with it, they are life-saving therapies.
Even outside oncology, predictive biomarkers are emerging. Rheumatoid arthritis patients with high rheumatoid factor levels respond better to rituximab, while those with inflammatory signatures may benefit from tocilizumab. In Alzheimer’s disease, genetic and inflammatory biomarkers point toward the JAK-STAT pathway as a therapeutic target.
These cases highlight a profound shift: diseases are no longer defined solely by organ or histology, but by molecular signatures that dictate treatment strategy.
Resistance and Relapse: The Other Side of the Coin
If predictive biomarkers guide us toward likely responders, resistance biomarkers warn us when treatments are doomed to fail.
Cancer provides countless examples. Mutations in KRAS, MET, or PI3K often herald resistance to trastuzumab in gastric cancer. Detecting these changes early allows clinicians to pivot strategies before wasting months on ineffective therapy.
Circulating tumor DNA (ctDNA) has revolutionized relapse monitoring. Fluctuations in ctDNA often precede radiological progression, making it an early harbinger of treatment failure. Similarly, donor-derived cell-free DNA in transplant medicine provides a sensitive, non-invasive biomarker of graft injury—detecting rejection before clinical deterioration occurs.
Even in diabetes, elevated fructosamine and glycated albumin signal resistance to insulin therapy, forewarning of vascular complications. Resistance biomarkers, in this sense, are not just scientific curiosities but pragmatic tools for clinicians battling therapeutic uncertainty.
Drug Toxicity: Protecting Patients Before It’s Too Late
Every drug is a double-edged sword—its benefits balanced by potential harms. Biomarkers provide the early warning system that tips this balance in favor of safety.
Traditional markers like ALT for liver function and creatinine for kidney injury remain staples. But modern pharmacology has expanded the repertoire. MicroRNA-122 is a sensitive biomarker of liver injury, correlating better with histological damage than conventional enzymes.
In cardiology, troponin levels detect chemotherapy-induced myocarditis or acute coronary syndrome long before clinical symptoms emerge. Similarly, regulatory agencies now require hERG channel assays and QTc interval studies to predict life-threatening arrhythmias from tyrosine kinase inhibitors.
Immunotherapy, the rising star of oncology, brings its own perils: immune-related adverse events. Elevated interleukin-6 not only predicts cytokine storms but also serves as a therapeutic target for IL-6 inhibitors—mitigating toxicity without blunting anti-cancer efficacy.
Here lies the beauty of safety biomarkers: they not only warn but sometimes suggest solutions.
Perspective: Where Do We Go from Here?
The trajectory of biomarkers in drug development is nothing short of transformative. They have evolved from simple laboratory measures into indispensable pillars of precision medicine. Yet challenges remain.
Standardization across assays is uneven, and biomarkers often capture only fragments of a disease’s complexity. Surrogate endpoints, while convenient, can mislead if not carefully validated. Moreover, the heterogeneity of human biology defies easy categorization.
Future directions are promising. Circulating DNA, microbiome profiling, proteomics, metabolomics, and artificial intelligence-driven biomarker systems promise to expand the toolkit. Composite biomarker panels, rather than single measures, may better capture the dynamic interplay of disease and therapy.
Most exciting is the prospect of biomarkers not only predicting or monitoring disease but becoming therapeutic levers themselves—as seen in gene therapy for hemophilia or IL-6 targeting in immunotherapy toxicity.
If drug development is a journey, biomarkers are both the map and the compass. They do not guarantee safe passage, but without them, we would be navigating blind.
FAQ
1. Why are biomarkers so critical in drug development?
Biomarkers provide measurable, objective parameters that accelerate drug development by identifying the right patients, confirming target engagement, predicting therapeutic response, and detecting toxicity early. Without them, trials would be longer, costlier, and riskier.
2. Can biomarkers replace clinical outcomes in drug approval?
Not entirely. While biomarkers often serve as surrogate endpoints to speed up trials, they must be rigorously validated. A biomarker may correlate with outcomes but does not always guarantee improved survival or quality of life.
3. What does the future hold for biomarkers in precision medicine?
Emerging technologies—such as ctDNA monitoring, microbiome profiling, and AI-driven multi-omics—promise to create integrated biomarker systems. These will allow not only earlier detection of disease and resistance but also highly individualized therapeutic strategies.


