Introduction
Organic erectile dysfunction (ED) has long been a clinical puzzle that stretches beyond the limits of oral pharmacotherapy. Although phosphodiesterase type 5 inhibitors (PDE5is) such as sildenafil and tadalafil revolutionized sexual medicine, their effectiveness is highly dependent on intact vascular and neurogenic mechanisms. For many men with diabetes, hypertension, pelvic surgeries, or profound endothelial damage, these drugs provide, at best, partial relief. The unmet medical need for therapies that do more than temporarily modify signaling cascades has inspired scientists to think outside the pillbox.
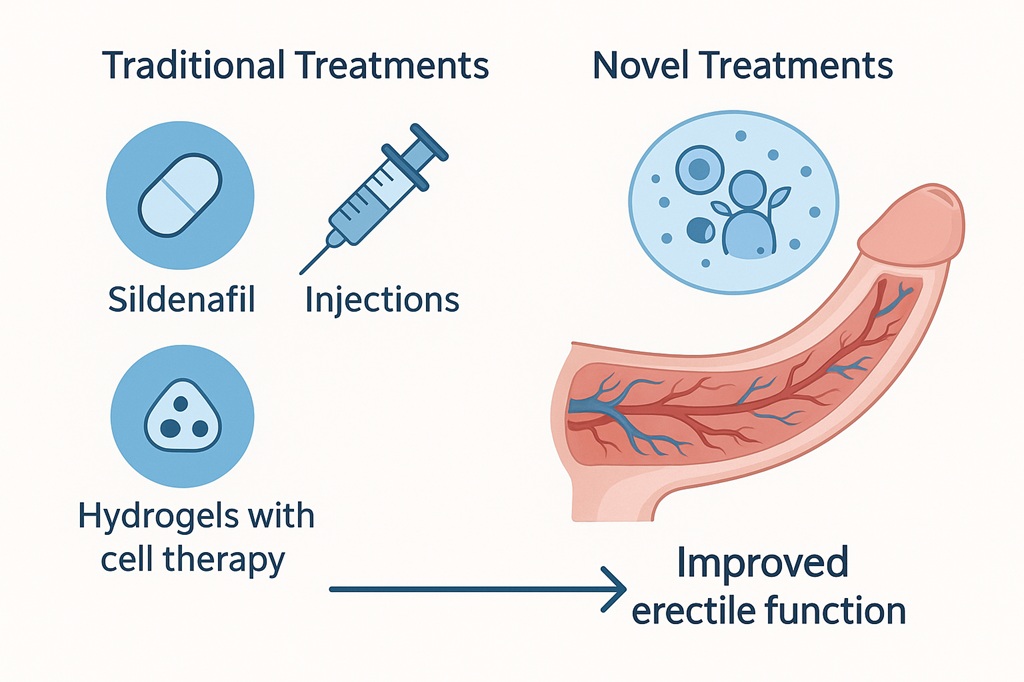
Among the many experimental strategies, hydrogels have emerged as a surprisingly elegant and promising approach. These water-rich, biocompatible polymeric networks provide not only structural support but also the ability to deliver drugs, genes, or stem cells directly to penile tissue in a controlled fashion. Unlike systemic therapies that must navigate hepatic metabolism and vascular clearance, hydrogel systems can be localized, sustained, and responsive to microenvironmental cues. The concept is ambitious: move from transient symptom management toward genuine penile tissue repair.
This article explores in depth how hydrogels, in their various advanced incarnations, may alter the trajectory of ED treatment. We will consider their design, biological interactions, mechanisms of action, and translational potential. With equal parts scientific rigor and cautious optimism, hydrogels invite us to reconsider what the future of sexual medicine might look like.
Understanding the Pathophysiology of Organic ED
Erectile function is a finely tuned interplay between neurovascular inputs, endothelial health, and smooth muscle relaxation. Organic ED arises when these mechanisms falter due to structural or metabolic insults. Diabetes mellitus, for instance, accelerates endothelial damage, reduces nitric oxide bioavailability, and stiffens cavernosal smooth muscle. Hypertension and atherosclerosis exacerbate arterial insufficiency, while radical pelvic surgery disrupts nerve supply. The result is a penis that has not forgotten how to respond but has lost the physical capacity to do so.
Conventional pharmacological solutions work by amplifying the residual nitric oxide–cyclic GMP pathway. In patients with intact vasculature, PDE5 inhibitors are highly successful. But when cavernous tissue is fibrotic, vessels are narrowed, and nerves are blunted, amplifying a broken signal does little. This explains the frustration many men experience: the therapy is scientifically sound yet practically ineffective in their specific condition.
What the field desperately requires is a method not only to potentiate existing signals but to rehabilitate tissue. True restoration of penile architecture — healthier vessels, improved endothelial function, and preserved smooth muscle — is the ambitious goal. This is where hydrogels, with their dual capacity for mechanical support and targeted bioactive delivery, become intriguing candidates.
Hydrogels: The Basics and Beyond
Hydrogels are three-dimensional polymeric structures that can absorb and retain large amounts of water, mimicking the extracellular matrix of natural tissues. They are soft, elastic, and tunable, properties that make them ideal candidates for integration into delicate organs such as the penis. The polymers used may be natural (e.g., collagen, alginate, chitosan) or synthetic (e.g., polyethylene glycol, polyvinyl alcohol), each with distinct degradation profiles and mechanical strength.
What makes hydrogels particularly fascinating is their versatility. They can be engineered to release drugs in response to specific stimuli — pH, temperature, or enzymatic activity — or to provide a sustained release over days to weeks. Unlike systemic drug administration, this localized delivery minimizes off-target effects and optimizes bioavailability exactly where it is needed.
Beyond being inert carriers, hydrogels can actively interact with tissues. By integrating growth factors, stem cells, or genetic material, they can serve as scaffolds for tissue regeneration. In cavernosal tissue compromised by diabetes or injury, a hydrogel scaffold may provide both the physical structure for repair and the biological signals to stimulate angiogenesis and neuroprotection.
Drug-Loaded Hydrogels: A Smarter Way to Deliver Therapy
The most straightforward application of hydrogels in ED therapy is as drug depots. Instead of swallowing a PDE5 inhibitor with uncertain systemic absorption, a hydrogel could deliver it directly to the corpora cavernosa. This approach has several advantages: rapid local action, reduced systemic side effects, and sustained presence of the drug at the target site.
Researchers have experimented with hydrogels loaded not only with PDE5is but also with vasoactive agents such as prostaglandin E1. Intracavernosal injections are notoriously disliked by patients, but a slow-release hydrogel could achieve the same pharmacological effect without repeated puncture trauma. Furthermore, controlled release profiles can reduce peaks and troughs in drug concentration, producing smoother physiological responses.
The broader implication is that localized drug delivery could transform compliance and efficacy. A patient who previously abandoned therapy due to headaches or hypotension might tolerate a hydrogel-based system that provides benefits without systemic burden. This seemingly simple modification of route and kinetics could redefine pharmacotherapy for ED.
Growth Factor and Gene Delivery via Hydrogels
Where hydrogels truly distinguish themselves is in their capacity to deliver molecules that cannot otherwise survive systemic circulation. Vascular endothelial growth factor (VEGF), nerve growth factor (NGF), and hepatocyte growth factor (HGF) have all demonstrated potential in preclinical models for promoting penile angiogenesis and neurogenesis. However, injected as free proteins, these factors are degraded quickly and may cause systemic complications.
Hydrogels provide a protective microenvironment for these labile molecules, releasing them gradually at the site of injury. For example, a VEGF-loaded hydrogel could encourage the sprouting of new vessels within the corpora cavernosa, directly addressing the ischemic component of organic ED. Similarly, NGF delivery could support the regeneration of cavernous nerves damaged during prostatectomy.
Gene therapy, often viewed with suspicion due to safety concerns, becomes more plausible when localized within a hydrogel scaffold. By embedding plasmid DNA or viral vectors into a biocompatible matrix, researchers can limit distribution to the penile tissue, reducing systemic exposure while prolonging local activity. Although far from ready for clinical practice, this strategy embodies the regenerative spirit of hydrogel research.
Stem Cell and Hydrogel Synergy
Stem cell therapy has long been touted as the holy grail of ED management, particularly for patients with diabetes or radical pelvic surgery. Mesenchymal stem cells (MSCs) can differentiate into endothelial or smooth muscle cells and secrete paracrine factors that improve vascularization. However, when injected directly into cavernosal tissue, most stem cells are quickly cleared, limiting their therapeutic efficacy.
Hydrogels solve this problem by providing a hospitable microenvironment where stem cells can survive, engraft, and function. Think of the hydrogel as both a protective cocoon and a nurturing soil. It retains the stem cells in place, shields them from hostile oxidative stress, and allows gradual interaction with host tissue.
Animal models have shown improved erectile responses when stem cells are delivered via hydrogels compared to direct injection. Importantly, the benefits appear longer-lasting, hinting at genuine tissue remodeling rather than transient paracrine effects. If this synergy translates into humans, it could mark a significant leap from symptomatic to restorative therapy.
Responsive Hydrogels: Precision in Action
Static drug release is useful, but intelligent, responsive hydrogels offer even greater promise. These systems are designed to alter their behavior in response to the penile microenvironment. For instance, in diabetic tissue with acidic pH and elevated reactive oxygen species, a hydrogel might release antioxidant molecules precisely when and where they are needed.
Temperature-sensitive hydrogels can undergo sol-to-gel transitions at body temperature, allowing minimally invasive injection in liquid form that solidifies upon contact with tissue. Enzyme-sensitive hydrogels degrade in response to matrix metalloproteinases, which are elevated in diseased tissues, thus releasing therapeutic payloads preferentially in damaged zones.
The elegance of these designs lies in their biomimicry. By listening to the biochemical whispers of the tissue, hydrogels act less like crude drug pumps and more like active collaborators in healing. This adaptive quality distinguishes them from the blunt instruments of systemic pharmacology.
Safety, Biocompatibility, and Ethical Considerations
Enthusiasm for hydrogels must be tempered by rigorous consideration of safety. Any material implanted into cavernosal tissue must not provoke chronic inflammation, fibrosis, or carcinogenesis. Natural polymers such as collagen and alginate are generally well tolerated but may degrade unpredictably. Synthetic polymers allow better control but can provoke foreign body responses if improperly engineered.
Sterility, reproducibility, and scalability are additional concerns. A hydrogel that works beautifully in a rodent study may prove difficult to manufacture consistently for thousands of patients. Furthermore, patients must accept the idea of penile injection or implantation, which requires education and reassurance. For many, the psychological barrier is as significant as the biological one.
Ethically, one must ask: are we creating permanent modifications of erectile tissue with unknown long-term consequences? The promise of restoration must be weighed against the risk of unintended outcomes. Until robust long-term human data emerge, hydrogels remain in the experimental domain, inspiring hope but demanding caution.
Translational Challenges and Clinical Horizons
Bridging the gap between laboratory innovation and clinical adoption is always the hardest step. Preclinical data on hydrogel-based ED therapies are encouraging, but translation into large human trials faces hurdles. Regulatory pathways for combination products — part device, part biologic, part drug — are notoriously complex.
Patient selection is another challenge. Not all men with ED require or would benefit from regenerative therapy. Identifying those with severe structural damage, refractory to conventional drugs, will be essential to demonstrate meaningful benefit. Moreover, endpoints in sexual medicine trials must move beyond crude metrics of erection hardness toward holistic measures of satisfaction and quality of life.
Despite these hurdles, the trajectory of hydrogel research is inspiring. A decade ago, the idea of repairing penile tissue with bioactive scaffolds seemed fanciful. Today, it is a legitimate research frontier attracting attention from biomaterials scientists, urologists, and pharmaceutical innovators alike.
Conclusion
Hydrogels offer a compelling vision for the future of erectile dysfunction therapy: a shift from symptomatic management to regenerative restoration. By serving as drug depots, growth factor reservoirs, gene delivery vehicles, and stem cell sanctuaries, they embody the convergence of material science and sexual medicine. Their responsiveness to tissue microenvironments adds a layer of sophistication unmatched by traditional approaches.
Yet it is vital to maintain perspective. These technologies remain in early stages of translation. Safety, scalability, patient acceptance, and regulatory approval are formidable barriers. For now, PDE5 inhibitors will continue to dominate clinical practice. But the very existence of hydrogel research testifies to the evolving ambition of sexual medicine: to not only enable erections but to rebuild the very tissue that makes them possible.
If successful, hydrogels could transform ED management from temporary assistance to durable recovery — a goal that is as bold as it is necessary. In a field where hope often collides with disappointment, hydrogels represent not fantasy but disciplined imagination in action.
FAQ
1. Are hydrogels for erectile dysfunction already available to patients?
Not yet. Most hydrogel-based therapies for ED remain in preclinical or early experimental stages. Human clinical trials are limited, and regulatory approval is still distant. Current treatment remains focused on oral PDE5 inhibitors and other established modalities.
2. How do hydrogel therapies differ from standard ED drugs?
Standard drugs like sildenafil work by amplifying existing biochemical signals. Hydrogels, in contrast, can deliver drugs, growth factors, or stem cells directly into penile tissue, with the aim of repairing damaged vasculature and nerves. The goal is not just symptom relief but genuine tissue restoration.
3. What are the main risks or concerns with hydrogel therapy?
Potential risks include tissue inflammation, fibrosis, infection, or unpredictable degradation of the hydrogel. Long-term safety data in humans are lacking. Ethical and psychological considerations regarding penile injections or implants also play a significant role in future acceptance.