Introduction
In medicine, certain molecules appear repeatedly in seemingly unrelated contexts—cardiovascular protection, cancer biology, neurological regulation, and immune modulation. One such molecule is adenosine, a humble purine nucleoside that quietly orchestrates countless cellular functions. If the body were an orchestra, adenosine would be the conductor: often unnoticed by the audience but essential for harmony.
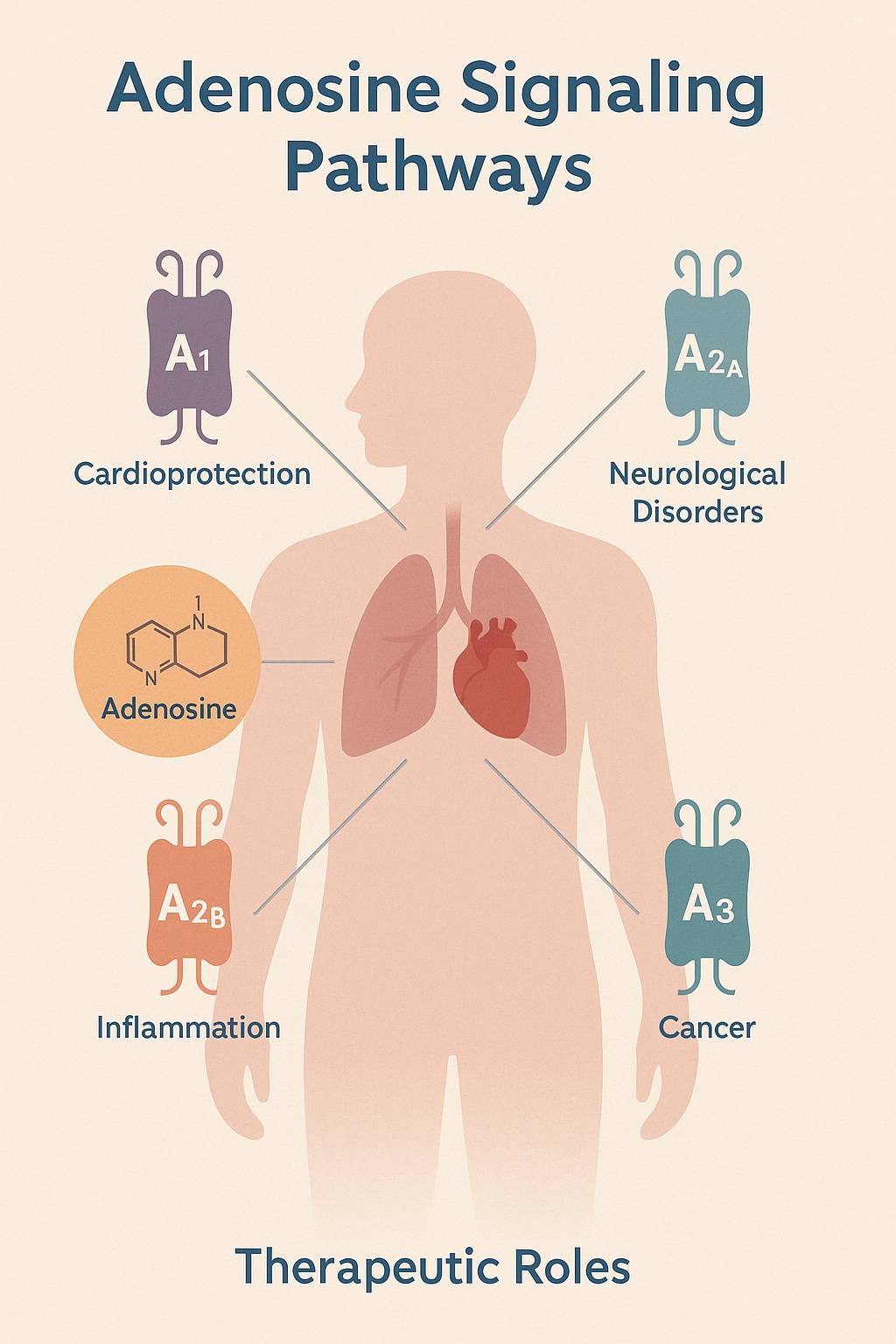
Once regarded as a mere by-product of ATP metabolism, adenosine is now recognized as a powerful signaling mediator with far-reaching influence. Its effects are mediated by a family of G protein–coupled receptors (A1, A2A, A2B, and A3), each distributed across tissues and linked to diverse physiological outcomes. The nuance lies in context: adenosine can be protective in one setting and harmful in another, much to the frustration of pharmacologists who seek clean therapeutic targets.
This article unpacks the science of adenosine signaling and explores how these pathways are being harnessed for therapeutic innovation. From ischemic heart disease to neurodegeneration, from cancer immunotherapy to chronic inflammation, adenosine offers both challenges and opportunities. By the end, the reader should appreciate why this modest nucleoside may well be one of the most versatile molecules in modern pharmacology.
The Biology of Adenosine
Adenosine arises from the breakdown of adenosine triphosphate (ATP), the cell’s energy currency. Under stress conditions such as hypoxia or ischemia, ATP consumption accelerates, and extracellular adenosine levels rise. This accumulation acts as a danger signal, alerting tissues to conserve energy and limit damage.
The molecule exerts its effects through four receptor subtypes:
- A1 receptors: generally inhibitory, reducing excitability in neurons and slowing conduction in the heart.
- A2A receptors: vasodilatory and immunomodulatory, promoting increased blood flow and anti-inflammatory signaling.
- A2B receptors: low affinity but important in high-adenosine states, often involved in inflammation and fibrosis.
- A3 receptors: less understood, implicated in cytoprotection and, paradoxically, tumor progression.
Importantly, receptor expression is tissue-specific and context-dependent. The same receptor may be beneficial in one organ yet deleterious in another, complicating drug development. This biological “double-edged sword” underscores the importance of nuanced therapeutic design.
Cardiovascular Implications
Few fields have embraced adenosine as enthusiastically as cardiology. Its role in controlling heart rate and vascular tone makes it a natural pharmacological tool. Clinicians are already familiar with intravenous adenosine as a first-line treatment for supraventricular tachycardia, where it induces a transient atrioventricular nodal block.
Beyond arrhythmias, adenosine contributes to ischemic preconditioning—the phenomenon whereby brief, non-lethal ischemic episodes render the heart more resistant to subsequent injury. Here, adenosine activates A1 and A3 receptors, reducing calcium overload, limiting oxidative stress, and improving mitochondrial resilience.
Therapeutically, the concept has led to investigations into adenosine analogs as cardioprotective agents during cardiac surgery or myocardial infarction. The challenge lies in balancing efficacy with side effects such as hypotension, bradycardia, and systemic vasodilation. While the “perfect” adenosine-based cardioprotective drug remains elusive, the pathway remains a fertile ground for innovation.
Neurological Dimensions
In the brain, adenosine operates as a neuromodulator, dampening excessive excitation and protecting neurons under stress. Its inhibitory actions at A1 receptors reduce neurotransmitter release, while A2A receptors in the basal ganglia modulate dopamine signaling.
Clinically, this duality is most evident in Parkinson’s disease. Overactivation of A2A receptors worsens motor symptoms, and accordingly, A2A antagonists such as istradefylline have been developed as adjunct therapies. These drugs improve motor function without the dyskinesia risk of higher-dose dopamine replacement.
Epilepsy is another area of interest. Adenosine’s ability to suppress neuronal excitability has inspired efforts to deliver it locally, including implantable adenosine-releasing polymers for refractory seizures. While still experimental, such approaches highlight adenosine’s therapeutic versatility.
At the same time, excessive adenosine may contribute to cognitive impairment and fatigue, particularly through prolonged A2A activation. Thus, the neurological applications of adenosine depend heavily on finding the right receptor, dose, and context.
Adenosine in Cancer: Friend or Foe?
Perhaps the most paradoxical role of adenosine lies in oncology. Within the tumor microenvironment, hypoxia drives high extracellular adenosine concentrations, which in turn suppress immune activity via A2A and A2B receptors. Cytotoxic T cells and natural killer cells, key players in antitumor defense, become “silenced” in this adenosine-rich milieu.
From this perspective, adenosine acts as a shield for cancer cells, blunting immune surveillance. This insight has spurred intense interest in adenosine receptor antagonists as immune checkpoint therapies. Early clinical trials suggest that blocking A2A signaling can reinvigorate T cell function, especially in combination with established immunotherapies like PD-1/PD-L1 inhibitors.
On the flip side, adenosine also has direct antiproliferative effects in certain cancers through A3 receptor activation. This receptor has been studied for potential anti-leukemic and anti-melanoma activity. Thus, the oncology field must navigate a complex balance: should we block adenosine to unleash immunity or activate it to suppress tumor growth? The answer depends on tumor type, stage, and microenvironmental context.
Inflammation and Immunity
Adenosine’s immunological profile is equally nuanced. Under acute stress, rising adenosine levels serve as a natural anti-inflammatory brake, preventing excessive tissue damage. Activation of A2A receptors dampens pro-inflammatory cytokine release, curbs neutrophil activation, and enhances tissue repair.
This effect is potentially valuable in diseases marked by runaway inflammation, such as sepsis, acute lung injury, and inflammatory bowel disease. In animal models, adenosine analogs reduce mortality from septic shock and ameliorate colitis symptoms.
However, in chronic settings, prolonged adenosine exposure may promote fibrosis, angiogenesis, and pathological tissue remodeling—particularly through A2B receptor activation. This duality illustrates once again the central paradox: adenosine is both guardian and saboteur, depending on context.
Therapeutic Strategies
Given adenosine’s vast reach, therapeutic approaches can be grouped into several categories:
- Receptor agonists: designed to mimic adenosine’s effects, useful in cardioprotection, acute ischemia, and seizure control.
- Receptor antagonists: block adenosine’s inhibitory actions, particularly valuable in oncology and Parkinson’s disease.
- Enzyme modulators: targeting CD39 and CD73, enzymes that generate extracellular adenosine, offers another lever for controlling signaling.
- Transport inhibitors: regulating nucleoside transport can alter extracellular adenosine availability.
Each approach comes with challenges. Agonists risk off-target hypotension or sedation; antagonists may unleash excessive inflammation; enzyme inhibitors can affect multiple cell types. The holy grail is selectivity—designing drugs that act precisely where needed, without collateral effects.
Challenges in Drug Development
Why, despite decades of research, do we not yet have a wide array of adenosine-based drugs in daily clinical use? The answer lies in the complexity of receptor distribution and the context-dependent nature of signaling.
First, receptor subtypes are not confined to single organs. An A2A antagonist designed to boost immune function may inadvertently increase cardiovascular risk. Second, adenosine’s short half-life in circulation complicates drug delivery. Achieving adequate tissue concentrations without systemic spillover is a constant struggle.
Furthermore, interindividual variability matters. Genetic polymorphisms in adenosine receptors, enzymes, and transporters influence response. Personalized medicine approaches may be required to fully exploit adenosine’s therapeutic potential.
Future Directions
The next chapter in adenosine therapeutics will likely focus on precision and synergy. Nanotechnology may allow targeted delivery of adenosine modulators to specific tissues, minimizing systemic exposure. Combination therapies—pairing adenosine antagonists with checkpoint inhibitors in cancer, or adenosine agonists with reperfusion therapies in cardiology—are already being tested.
Biomarker development is also crucial. If clinicians can reliably measure adenosine levels or receptor activation states in patients, they could tailor therapy with unprecedented precision. Advances in imaging and metabolomics may soon make this feasible.
Ultimately, adenosine represents a master regulator of physiology. Learning to manipulate it safely could transform multiple fields simultaneously. The path is challenging, but the reward—therapeutic leverage across cardiovascular disease, neurology, oncology, and immunology—is enormous.
Conclusion
Adenosine has traveled a remarkable journey: from a metabolic footnote to a central signaling molecule with therapeutic promise across nearly every branch of medicine. It embodies the paradox of biology—protector and perpetrator, healer and accomplice.
The challenge for medicine is not whether adenosine matters (it clearly does) but how to harness its power without unleashing its darker side. With advances in receptor-selective drugs, delivery technologies, and systems biology, the dream of adenosine-targeted therapy is inching closer to reality.
For now, adenosine remains a tantalizing universal key—one that may yet unlock new frontiers in healing.
FAQ
1. Why is adenosine called a “universal regulator”?
Because it influences so many systems—heart rhythm, blood vessels, immune response, and brain signaling. Its receptors are widely expressed, giving it broad physiological reach.
2. Are there already drugs targeting adenosine?
Yes. Intravenous adenosine is used for arrhythmias, and A2A antagonists are approved for Parkinson’s disease. Several experimental drugs are in development for cancer, inflammation, and cardioprotection.
3. What is the main obstacle to adenosine-based therapy?
The biggest challenge is context. The same receptor can be protective in one disease and harmful in another. Achieving precise targeting without side effects remains the central hurdle.