Adenosine, a seemingly humble nucleoside, is in reality a master conductor of cellular communication, influencing cardiovascular function, immune responses, neural activity, and cancer progression. Over the past decades, biomedical research has revealed that adenosine is more than a metabolic byproduct—it is a key regulator, capable of protecting tissues under stress, modulating inflammation, and, paradoxically, fueling tumor growth under certain conditions.
This article explores the therapeutic world of adenosine and its receptors. It provides a comprehensive review of how adenosine signaling is being targeted in modern medicine, with a focus on cardiovascular and neurological disorders, cancer, immune regulation, and beyond. At the same time, we will not shy away from irony: while adenosine protects life, it can also be a cunning accomplice in disease.
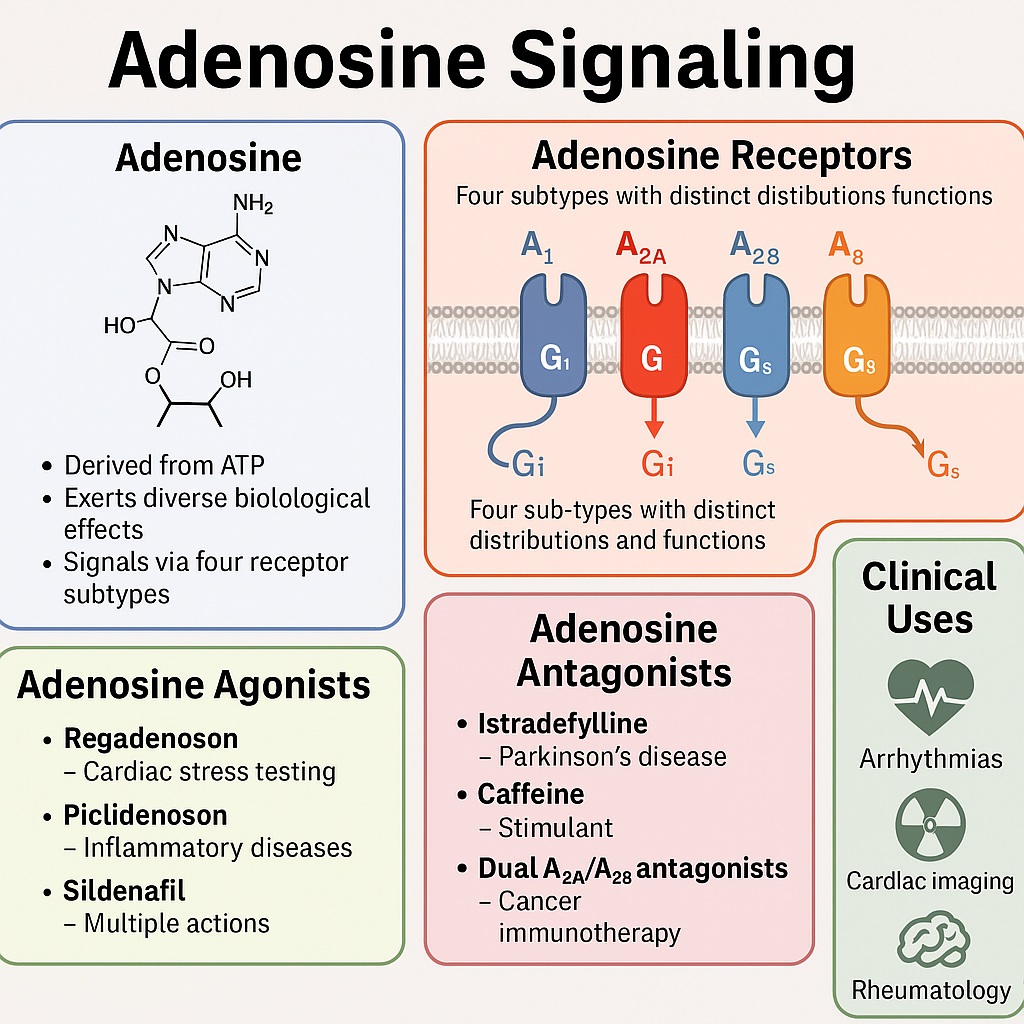
Adenosine: The Cellular Peacemaker
Adenosine arises from the breakdown of ATP, the cell’s primary energy currency. When tissues experience metabolic stress—such as ischemia, hypoxia, or inflammation—adenosine concentrations rise in the extracellular space. This surge acts as a distress signal, engaging specific receptors on cell surfaces to restore balance.
There are four main adenosine receptor subtypes: A1, A2A, A2B, and A3. Each receptor couples to different G-proteins, triggering diverse intracellular cascades. The A1 and A3 receptors typically inhibit cyclic AMP (cAMP) production, whereas A2A and A2B receptors enhance it. This yin-yang system allows adenosine to fine-tune vascular tone, neurotransmission, and immune activity.
Adenosine is often called a “cellular peacemaker” because of its protective role. In the heart, it reduces oxygen demand by slowing conduction and dilating coronary vessels. In the immune system, it tempers inflammation by suppressing T-cell activity and neutrophil activation. However, this same peacekeeping capacity can turn treacherous: cancer cells exploit adenosine to suppress immune surveillance, creating a shield against destruction.
Therapeutic Adenosine: Old Drug, New Tricks
Clinicians have long recognized adenosine’s therapeutic potential. Intravenous adenosine is a first-line treatment for supraventricular tachycardia, where it briefly halts atrioventricular conduction, restoring normal rhythm. Beyond this, adenosine stress tests are a cornerstone of cardiac imaging, exploiting its vasodilatory effects to reveal coronary artery disease.
Yet, researchers have realized that adenosine is not merely an acute intervention tool—it is a gateway to an entire pharmacological universe. Drugs can be designed to either mimic adenosine (agonists) or block its actions (antagonists). Moreover, medications with seemingly unrelated targets—such as methotrexate, aspirin, and even sildenafil—have been discovered to exert part of their effects through adenosine pathways.
This revelation has two consequences. First, it provides a rationale for drug repurposing, allowing old medicines to find new uses. Second, it challenges us to refine adenosine modulation with precision, avoiding the pitfalls of global immunosuppression or excessive vasodilation.
Adenosine Antagonists: Awakening the Nervous System
One of the best-known adenosine antagonists is caffeine. This everyday stimulant blocks A2A receptors in the brain’s basal ganglia, counteracting the inhibitory influence of adenosine on dopamine signaling. The result is enhanced alertness, reduced fatigue, and improved motor performance—a pharmacological trick humanity has enjoyed for centuries without knowing the molecular details.
In neurology, selective A2A antagonists have found their place in Parkinson’s disease. Istradefylline, an A2A receptor blocker, was approved in 2019 to alleviate “wearing-off” episodes in patients receiving levodopa. Unlike caffeine, istradefylline provides targeted modulation, improving motor control without the jitteriness of excess coffee.
This raises an intriguing irony: adenosine, usually celebrated as a protector against cellular injury, becomes an adversary in Parkinson’s disease by dampening dopamine signaling. By blocking adenosine’s restraining hand, we restore movement—a prime example of context defining virtue or vice.
Adenosine Agonists: Harnessing Protection
If antagonists awaken the nervous system, agonists calm and protect. Regadenoson, a selective A2A receptor agonist, has largely replaced adenosine in cardiac stress testing. Its bolus administration is more convenient and better tolerated. Beyond diagnostics, regadenoson is under investigation for treating ischemia-reperfusion injury and even mitigating hyperinflammation in severe COVID-19.
Sildenafil, better known for erectile dysfunction and pulmonary hypertension, indirectly activates adenosine pathways. Studies suggest its antinociceptive and cardioprotective effects involve A2A receptor signaling. Once again, adenosine proves to be the “hidden hand” behind pharmacology.
Agonists at the A3 receptor subtype are showing promise in inflammatory and oncological settings. Piclidenoson, for instance, has demonstrated anti-inflammatory activity in rheumatoid arthritis and inflammatory bowel disease, though clinical superiority over methotrexate remains elusive. The A3 receptor may also serve as a biomarker, as higher receptor expression correlates with therapeutic response.
Dual Antagonists: Blocking Adenosine’s Dark Side
While adenosine protects normal tissues, it can also suppress immune responses in the tumor microenvironment. Cancer cells exploit adenosine to evade immune destruction, creating an immunosuppressive shield. This has led to the development of dual A2A/A2B antagonists, such as etrumadenant, which restore T-cell activity and enhance CAR-T therapy.
Clinical trials are underway to test these agents in combination with checkpoint inhibitors and chemotherapy. The strategy is elegant: by unmasking tumors from adenosine’s cloak, the immune system regains its natural power. It is as if adenosine, once a peacekeeper, is forcibly retired when it sides with malignancy.
Adenosine and Classic Anti-Inflammatory Drugs
Many nonsteroidal anti-inflammatory drugs (NSAIDs) exert part of their effects through adenosine signaling. Salicylates, for example, increase extracellular adenosine by uncoupling oxidative phosphorylation. Methotrexate, the cornerstone of rheumatoid arthritis therapy, enhances adenosine production via CD73 upregulation and inhibition of AICAR transformylase.
This adenosine-mediated mechanism explains methotrexate’s rapid anti-inflammatory benefits, distinct from its anti-proliferative action. It also accounts for paradoxical flare-ups upon drug discontinuation, as the protective adenosine brake is suddenly lifted.
In essence, many old drugs we thought we understood are quietly whispering through adenosine. Recognizing this expands therapeutic insight and encourages refinement.
CD73 Inhibitors: Cutting the Supply Line
Adenosine is generated from extracellular AMP via the enzyme CD73. Inhibiting this step is an attractive anti-cancer strategy. Monoclonal antibodies and small-molecule CD73 inhibitors such as quemliclustat are now in clinical trials. By cutting off adenosine production, these drugs deprive tumors of their immunosuppressive weapon.
The challenge lies in selectivity: systemic blockade risks undermining adenosine’s protective roles in other tissues. Clinical outcomes so far are mixed, highlighting the complexity of balancing immune activation against collateral damage.
Cardiovascular Horizons: Adenosine’s Original Stage
In cardiology, adenosine remains indispensable. Beyond arrhythmia management and stress testing, research continues into its role in ischemia-reperfusion injury, heart failure, and pulmonary hypertension. Experimental studies show that adenosine receptor agonists can limit infarct size, reduce oxidative stress, and improve endothelial function.
Yet, translating these findings into clinical practice has been challenging. Human trials often fall short of the dramatic results seen in animals. The culprit may be timing, dosing, or the sheer complexity of human disease. Nonetheless, adenosine remains a promising cardioprotective target, waiting for the right strategy to unlock its full potential.
The Irony of Adenosine: Protector and Accomplice
Adenosine embodies medical irony. In ischemia, it protects the heart; in cancer, it protects the tumor. In the immune system, it prevents overactivation; in autoimmunity, this is welcome, but in infection, it may be detrimental. In neurology, blocking adenosine relieves Parkinson’s symptoms, yet stimulating it may protect against excitotoxic injury.
This duality reminds us that biology rarely provides simple heroes or villains. Instead, context defines morality. Adenosine is a peacemaker when balance is needed, and an accomplice when exploited by disease.
Future Directions: Precision Adenosinergic Medicine
The next frontier is precision modulation of adenosine signaling. Advances in receptor subtype selectivity, tissue-targeted delivery, and biomarker-guided therapy hold the promise of maximizing benefits while minimizing risks.
For example, A3 receptor expression may predict response to piclidenoson in arthritis. Tumor adenosine signatures could guide CD73 inhibitor use. Cardioprotective strategies may rely on timed adenosine agonism during reperfusion. Such precision will transform adenosine therapeutics from blunt instruments into finely tuned tools.
The field also illustrates the value of drug repurposing. From caffeine to sildenafil, many widely used drugs have hidden adenosine links. Exploring these connections offers cost-effective therapeutic innovation.
Conclusion
Adenosine is not a mere bystander of metabolism but a central regulator of human physiology and pathology. Its signaling pathways are being actively harnessed across cardiology, neurology, oncology, immunology, and rheumatology. While its dual nature poses challenges, it also opens opportunities for highly tailored interventions.
Targeting adenosine represents both a therapeutic challenge and a fascinating journey through biology’s paradoxes. The story is far from finished, but one lesson is clear: when cells whisper in adenosine, medicine must learn to listen carefully.
FAQ
1. Why is adenosine important in medicine?
Adenosine regulates blood flow, immune responses, and neural activity. It is used clinically to treat arrhythmias and in cardiac stress testing. Its receptors are therapeutic targets in cancer, Parkinson’s disease, rheumatoid arthritis, and more.
2. Are adenosine drugs safe for long-term use?
Safety depends on the drug and target. Acute use of adenosine for arrhythmias is safe, but chronic modulation requires caution. Selective receptor drugs (like istradefylline or regadenoson) improve tolerability, while broad-spectrum modulation risks side effects.
3. Will adenosine therapies become standard for cancer treatment?
Possibly. Trials of CD73 inhibitors and dual A2A/A2B antagonists show promise, especially in combination with immunotherapy. However, results are still preliminary, and balancing efficacy with safety remains a key challenge.