Introduction
Erectile physiology may appear straightforward at first glance—nitric oxide diffuses into smooth muscle, activates guanylyl cyclase, increases cyclic nucleotides, relaxes corporal tissue, and an erection follows. Yet this elegant cascade is only a simplified version of what actually occurs in cavernosal neurobiology. A modern and more precise understanding acknowledges that erection is orchestrated not by a single molecule but by a network of gasotransmitters, cyclic nucleotide pathways, ion channels, and neuromodulators that cooperate to permit penile tumescence. Among these, nitric oxide (NO), hydrogen sulfide (H₂S), and carbon monoxide (CO) play central roles, acting synergistically to regulate smooth muscle relaxation within the corpus cavernosum.
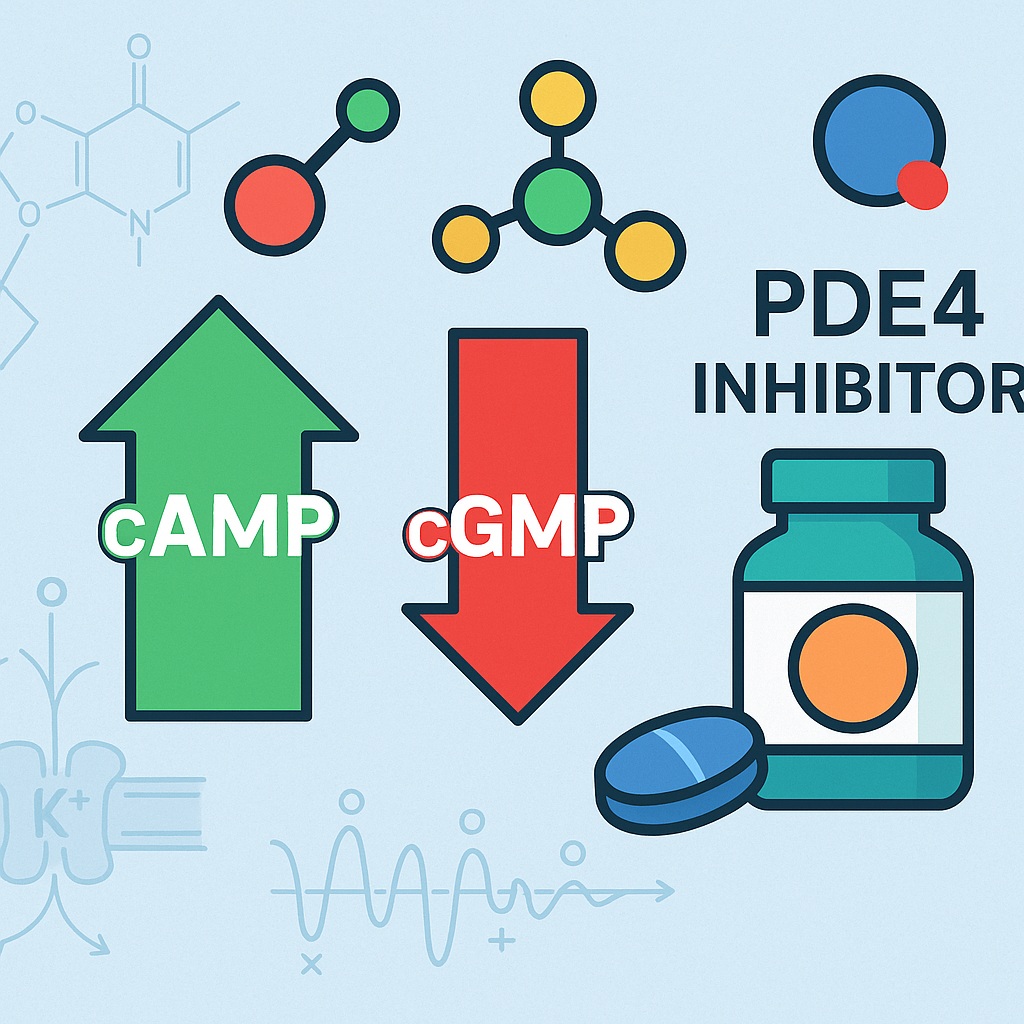
The study provided in the PDF explores an important and previously underappreciated regulatory mechanism within this system: the inhibition of phosphodiesterase type 4 (PDE4). While PDE5 has long been the therapeutic superstar in erectile dysfunction (ED), emerging evidence indicates that PDE4—primarily associated with cyclic AMP metabolism—may significantly influence cavernosal relaxation, particularly in pathways involving gasotransmitters.
This article synthesizes the study’s experimental findings with the current scientific understanding of cavernosal physiology. It critically examines how PDE4 inhibition enhances nerve-mediated relaxation induced by NO, H₂S, and CO, and explores the translational implications for future ED therapeutics. Beyond the biochemistry, we consider the broader message: erectile physiology is far more dynamic than the PDE5-centric model suggests, and recognizing this complexity opens the door to completely new therapeutic strategies.
The Role of Gasotransmitters in Erectile Physiology
To appreciate the significance of PDE4 inhibition, one must first understand the gasotransmitters involved in penile erection. The PDF clearly highlights three principal molecules—NO, CO, and H₂S—each functioning as a signaling gas capable of diffusing across membranes without the need for classical receptors.
Nitric Oxide (NO)
NO is the well-established mediator of cavernosal relaxation. Upon release from nitrergic neurons, it stimulates soluble guanylyl cyclase (sGC), increases cyclic guanosine monophosphate (cGMP), and causes smooth muscle relaxation. This pathway forms the backbone of current ED pharmacotherapy.
Hydrogen Sulfide (H₂S)
H₂S, produced via cystathionine β-synthase and cystathionine γ-lyase, has gained attention for its ability to activate KATP channels and promote cavernosal relaxation. The study confirms that H₂S-induced relaxation is enhanced under PDE4 inhibition, underscoring its relevance in ED research.
Carbon Monoxide (CO)
Though less discussed clinically, CO is produced via heme oxygenase and activates sGC similarly to NO. The study demonstrates that CO-mediated nerve responses are significantly potentiated by PDE4 inhibition.
Collectively, these gasotransmitters represent an intricate regulatory system. The PDF’s experimental graphs (pages 4–6) visually highlight how each gasotransmitter independently contributes to modulating neurogenic relaxation in the rat corpus cavernosum. Understanding their interplay is foundational to interpreting PDE4-mediated enhancements.
Why PDE4? Understanding the Enzyme’s Mechanistic Significance
Most sexual medicine research focuses on PDE5, the enzyme responsible for degrading cGMP. But penile smooth muscle contraction and relaxation also depend heavily on cyclic AMP (cAMP) pathways, which are regulated by PDE4.
According to the study, PDE4 enzymes are abundantly expressed in the corpus cavernosum, where they hydrolyze cAMP and modulate nerve-mediated responses. Importantly, several neuronal and endothelial gasotransmitter pathways—including those involving VIP (vasoactive intestinal peptide) and H₂S—use cAMP as a co-messenger or modulator.
Thus, PDE4 inhibition amplifies cAMP-dependent relaxation, which then synergizes with gasotransmitter activity.
The study’s core hypothesis, confirmed in its experimental data, is that PDE4 inhibitors can significantly potentiate:
- nitrergic nerve–mediated relaxation (NO)
- CO-induced relaxation
- H₂S-induced relaxation
These effects occur because PDE4 inhibition removes a metabolic “brake” on cyclic nucleotide accumulation. For erectile physiology, this means smoother neurovascular coupling, enhanced relaxation, and greater potential for erection.
Experimental Design and Methodology: Rat Corpus Cavernosum in Focus
The researchers used isolated rat corpus cavernosum strips, a classical model for studying erectile physiology. The tissues were electrically stimulated to induce nerve-mediated relaxation, with increasing frequencies (5–40 Hz) used to evaluate frequency-dependent changes.
Key experimental interventions included:
- PDE4 inhibitor rolipram
- sGC inhibitor ODQ (to confirm involvement of the NO–cGMP pathway)
- Potassium channel blockers
- Exogenous gasotransmitters (NaHS for H₂S, CO donor compounds)
The PDF’s methodology section (pages 2–3) details how tissues were precontracted with phenylephrine, creating a controlled baseline from which relaxation responses could be accurately measured.
Graphical data (Figures 2–6) add visual support for the conclusion that PDE4 inhibition substantially enhances nerve-mediated responses across all gasotransmitters studied.
Key Findings: PDE4 Inhibition Enhances Neurogenic Relaxation
1. PDE4 Inhibition Potentiates Nerve-Mediated Corpus Cavernosum Relaxation
One of the most important results appears in Figure 2 (page 4), showing that rolipram dramatically increases the amplitude of frequency-dependent relaxation. At 20–40 Hz, the enhancement becomes particularly pronounced—indicating a strong facilitation at higher levels of neural stimulation.
This suggests that during sexual arousal, when cavernosal nerves fire rapidly, PDE4 inhibition may provide maximal benefit.
2. Enhancement Across All Three Gasotransmitter Systems
The study demonstrates a consistent pattern:
- NaHS-induced relaxation (H₂S) is significantly enhanced under PDE4 inhibition (Figure 4).
- CO donor-induced relaxation is also amplified (Figure 5).
- Nitrergic relaxation, already a dominant physiological mechanism, shows robust potentiation.
This generalized enhancement implies that PDE4 inhibition does not target a single pathway but instead creates a permissive biochemical environment for multiple relaxation mechanisms.
3. Mechanistic Confirmation via Signal Pathway Blockers
The use of ODQ (sGC inhibitor) sharply decreased the PDE4-mediated potentiation of relaxation, confirming the involvement of cyclic nucleotide–dependent pathways.
Similarly, potassium channel blockers reduced relaxant responses, reinforcing the idea that membrane hyperpolarization is a key downstream effect.
Collectively, these findings provide mechanistic clarity and rule out non-specific effects.
Physiological Interpretation: Why PDE4 Inhibition Amplifies Gasotransmitter Effects
To understand why all three gasotransmitters benefited from PDE4 inhibition, we revisit the interplay between cAMP and cGMP systems.
Synergy Between Cyclic Nucleotide Pathways
Many gasotransmitters use interdependent pathways:
- NO → cGMP ↑
- CO → cGMP ↑
- H₂S → KATP activation + cAMP enhancement
- VIP neuronal pathways → cAMP ↑
By inhibiting PDE4, intracellular cAMP levels rise. Elevated cAMP indirectly potentiates cGMP-dependent relaxation, creating a synergistic amplification. Penile smooth muscle is exquisitely sensitive to changes in cyclic nucleotide concentrations, making this interaction physiologically significant.
Impact on Membrane Hyperpolarization
H₂S and other mediators activate potassium channels (especially KATP and BKCa channels), leading to hyperpolarization and relaxation. PDE4 inhibition increases the likelihood of channel activation by shifting intracellular signaling dynamics.
Interaction With Nitrergic Signaling
NO signaling remains dominant in erection. PDE4 inhibition strengthens NO effects not by interfering with PDE5 but by enhancing NO’s ability to produce robust relaxation via cross-talk between cAMP and cGMP systems.
The study provides compelling evidence that PDE4 inhibition does not merely “assist” gasotransmitters—it amplifies their fundamental mechanisms.
Translational Implications: Could PDE4 Become a Novel ED Target?
Perhaps the most exciting question is whether PDE4 inhibitors could serve as therapeutic agents for erectile dysfunction. While currently used for conditions such as COPD (e.g., roflumilast), they have not been explored clinically for ED.
This study suggests several potential benefits:
- PDE4 inhibitors may improve erections in men with poor response to PDE5 inhibitors, particularly those with neuropathic ED or impaired nitrergic function.
- They may provide a new therapeutic option for diabetic ED, where both NO and H₂S pathways are compromised.
- Their multi-gasotransmitter synergy may outperform PDE5 inhibition alone in certain mechanistic contexts.
However, PDE4 inhibitors are often associated with nausea and gastrointestinal side effects—an obstacle requiring pharmaceutical refinement.
The authors’ findings also hint at combination therapy possibilities: PDE5 + PDE4 inhibitors could theoretically maximize both cGMP and cAMP signaling, though such combinations would need careful safety evaluation.
Limitations Highlighted by the Study
No scientific study is without limitations, and the authors acknowledge several important constraints:
- In vitro model limitations: Rat tissue does not fully replicate human erectile physiology.
- Use of isolated gasotransmitter donors: Physiological release dynamics may differ in vivo.
- Rolipram’s side effect profile: Makes it unsuitable for direct clinical translation without modification.
- Indirect evidence: The study does not examine PDE4 expression distribution at the cellular level.
These limitations do not diminish the study’s value; rather, they highlight where future research must focus.
A Broader Perspective: Rethinking Erection Biology
This study serves as a reminder that erectile physiology is governed by a symphony of biochemical signals—not a solo act conducted by NO alone. Gasotransmitters complement each other, cyclic nucleotide pathways interact intimately, and phosphodiesterases regulate more than one signal.
For too long, ED research has focused almost exclusively on enhancing cGMP via PDE5 inhibition. The present findings encourage a paradigm shift: exploring cAMP-driven mechanisms, targeting novel PDE families, and leveraging the synergy between multiple gasotransmitters.
Such an approach promises more tailored, mechanistically precise treatments for patients who do not respond to existing therapies.
Conclusion
The study “In vitro inhibition of phosphodiesterase type 4 enhances rat corpus cavernosum nerve-mediated relaxation induced by gasotransmitters” represents a meaningful step toward expanding our understanding of erectile biology beyond the confines of the traditional NO–PDE5 model. By demonstrating that PDE4 inhibitors significantly enhance nerve-mediated relaxation induced by NO, H₂S, and CO, the researchers reveal a powerful amplification mechanism within cavernosal physiology.
This discovery lays the groundwork for new pharmacotherapeutic strategies centered on cyclic AMP modulation and invites a reconsideration of how multiple gasotransmitters cooperate to regulate erectile function. While PDE4 inhibitors in their current form are not ready for clinical use in ED, the concept is scientifically robust and represents a promising frontier for future research.
Ultimately, the study illuminates the complex biochemical choreography underlying erection and challenges the field to envision a broader, more integrated approach to treating erectile dysfunction.
FAQ
1. Why is PDE4 inhibition important for erectile physiology?
PDE4 regulates cAMP levels in cavernosal tissue. Its inhibition amplifies cAMP-dependent relaxation and synergizes with NO, CO, and H₂S pathways, enhancing nerve-mediated cavernosal relaxation.
2. Could PDE4 inhibitors be used clinically for erectile dysfunction?
Not yet. Existing PDE4 inhibitors have significant side effects. However, the mechanistic evidence suggests strong therapeutic potential once safer PDE4-selective compounds are developed.
3. How do gasotransmitters contribute to erection?
NO activates cGMP pathways, H₂S opens potassium channels and enhances cAMP, and CO stimulates sGC. Together, they coordinate smooth muscle relaxation in the corpus cavernosum. PDE4 inhibition strengthens the effects of all three.