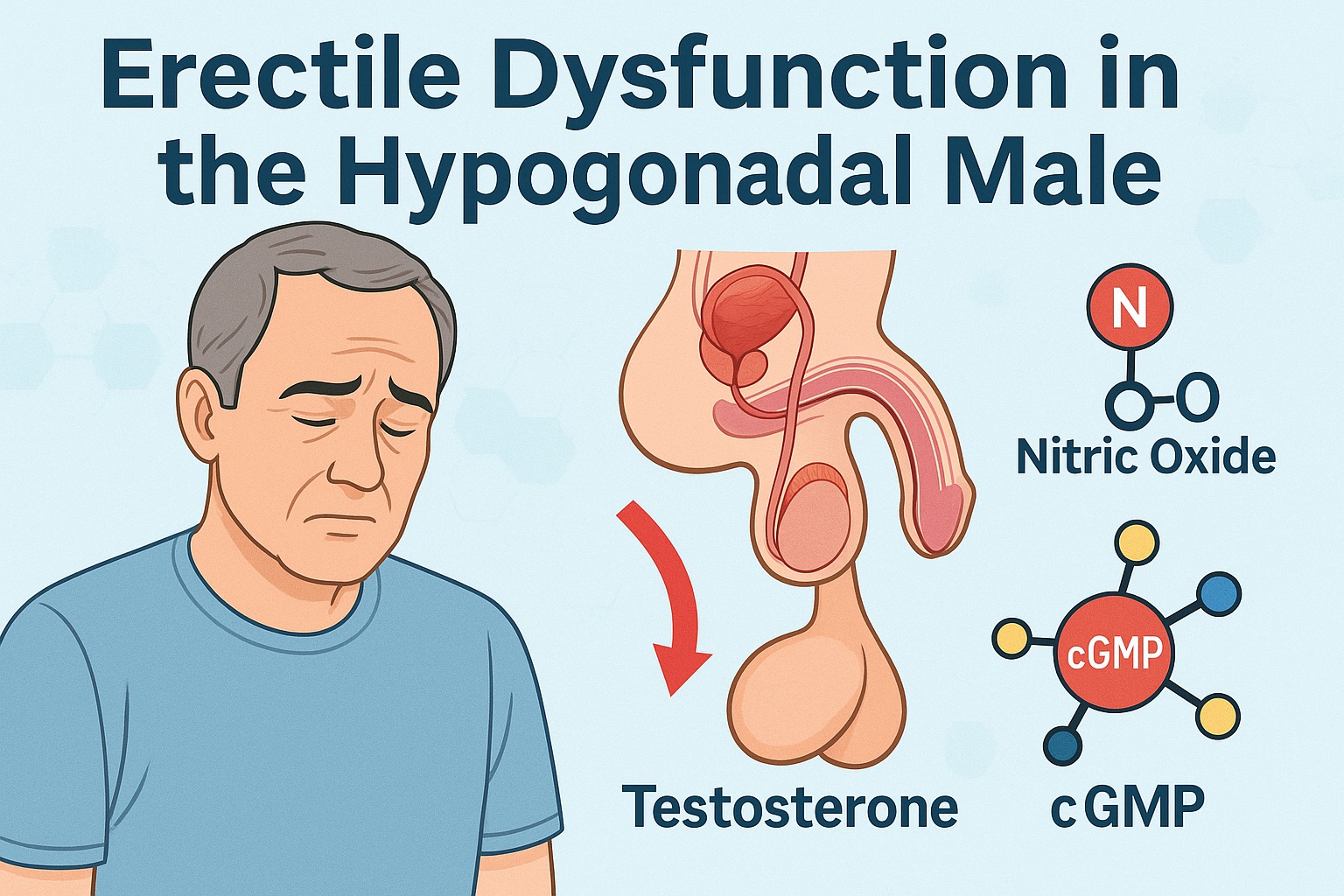
Erectile dysfunction (ED) in men with hypogonadism represents a particularly intricate clinical scenario—one in which endocrine insufficiency intersects with vascular impairment, psychological burden, and pharmacological resistance. Although phosphodiesterase-5 (PDE5) inhibitors such as sildenafil revolutionized ED management more than two decades ago, their efficacy is far from universal. In hypogonadal men, ED therapy becomes a more nuanced process, demanding an understanding not only of penile hemodynamics but also of androgen-dependent nitric oxide (NO) physiology. The clinical case at the heart of the referenced study demonstrates this complexity with unusual clarity. It guides us through failed trials, diagnostic revelations, hormone replacement, and, ultimately, the restoration of sexual function—illustrating why testosterone remains an indispensable cofactor in PDE5 inhibitor responsiveness.
The larger lesson is unmistakable: erectile function cannot be divorced from hormonal balance. Even the most potent PDE5 inhibitor cannot compensate for inadequate testosterone levels; molecular physiology simply refuses to cooperate. This article distills the case study into a broader exploration of mechanisms, diagnostic strategy, and therapeutic insights relevant for clinicians who routinely manage sexual dysfunction in hypogonadal patients.
Understanding the Hypogonadal Landscape: Why Low Testosterone Disrupts Erections Before the Patient Notices Anything Else
Hypogonadism does not announce itself abruptly. Instead, testosterone decline subtly erodes libido, nocturnal erections, energy levels, and sexual confidence until the patient eventually presents with what appears to be “just ED.” The truth is rarely that simple. Testosterone is central to several key aspects of erectile physiology: it maintains nitric oxide synthase (NOS) expression in penile nerves, regulates PDE5 activity, supports sexual desire, and modulates cavernosal smooth-muscle responsiveness. When androgen levels fall below physiologic thresholds, the NO cascade falters, vasodilation becomes inadequate, and PDE5 inhibitors lose much of their pharmacologic impact.
This biological dependency explains why many hypogonadal men fail initial trials of sildenafil. The drug’s mechanism requires intact NO production; sildenafil cannot create nitric oxide where there is none. In clinical practice, we often meet patients who tried sildenafil incorrectly, inconsistently, or without proper instruction. Yet an equally large cohort consists of men whose hormonal milieu simply cannot support PDE5 inhibitor efficacy.
The case described in the source text highlights this elegantly. The patient’s symptoms—reduced libido, diminishing erection quality, and unsatisfactory response to sildenafil—paint the classic picture of hypogonadal ED. Notably, even his wife observed a decline in sexual interest before the more obvious erectile symptoms appeared. Testosterone insufficiency disrupts both desire and performance, shaping the couple’s sexual dynamic long before ED becomes the primary complaint. Indeed, libido decline is often the earliest and most reliable clinical indicator of androgen deficiency, even when erectile capacity remains partially intact.
Clinical Evaluation: Why Good Diagnostics Matter More Than Rushing to the Prescription Pad
The diagnostic process in ED is often oversimplified in practice, with many men receiving PDE5 inhibitors before any evaluation of hormonal or vascular status. Yet testosterone deficiency and penile vascular disease contribute significantly to poor treatment response. A methodical approach remains essential.
The patient in the study underwent a comprehensive clinical history, including relationship dynamics, frequency of sexual activity, psychosocial stressors, lifestyle risk factors, and comorbidities. His medical background—mild hypertension, a remote smoking history, occupational stress—was not unusual. What was unusual was the absence of any prior endocrine or vascular evaluation despite multiple failed ED treatments.
His International Index of Erectile Function (IIEF) score confirmed moderate dysfunction, and further conversation revealed incorrect use of sildenafil. This scenario—improper dosing, administration with food, inadequate number of attempts—is so common that many “treatment failures” are really “instruction failures.” The clinician appropriately recommended a structured sildenafil rechallenge with proper guidance before moving to more invasive testing.
Nonetheless, considering the patient’s symptoms and poor initial response, laboratory diagnostics were performed. His testosterone level of 218 ng/dL was unequivocally low, and a second confirmatory measurement was even lower. Free testosterone was below normal as well, adding biochemical weight to the clinical suspicion of hypogonadism.
Importantly, duplex Doppler penile ultrasound (DUS) provided additional clarity. The patient demonstrated borderline arterial insufficiency—velocities below the 30 cm/s threshold—indicating compromised cavernosal inflow. This level of vascular impairment often coexists with systemic endothelial dysfunction, which may precede coronary artery disease. Indeed, ED can serve as an early warning sign of cardiovascular pathology, a link that clinicians must always keep in mind.
Thus, the diagnostic conclusion was multilayered: hypogonadism contributing to poor libido and erectile quality, combined with cavernosal arterial insufficiency diminishing erectile rigidity, compounded by suboptimal sildenafil responsiveness.
Testosterone’s Role in NO Signaling: The Molecular Explanation Behind PDE5 Inhibitor Failure in Hypogonadal Men
The most compelling aspect of the case is the mechanistic rationale behind sildenafil’s failure prior to testosterone supplementation. Nitric oxide synthase (NOS) expression in penile nerves is androgen dependent; when testosterone levels fall, NOS expression declines and NO availability decreases. Sildenafil’s mechanism—blocking PDE5 to prolong cGMP levels—requires adequate NO production to initiate the erection cascade. Without an adequate upstream signal, the downstream modulation remains futile.
Experimental models reinforce this reality. Castrated animals show drastically reduced NOS expression and impaired erectile response; androgen replacement restores both. Moreover, PDE5 expression itself is modulated by testosterone, suggesting that the pharmacodynamic environment of sildenafil is—and must be—hormonally optimized for the drug to function fully.
Testosterone’s involvement extends beyond NO signaling. It maintains cavernosal smooth muscle integrity, modulates vascular responsiveness, and supports libido—each a crucial component of erectile function. Consequently, a hypogonadal patient may present with:
- low NO availability,
- reduced PDE5 expression,
- impaired smooth muscle relaxation,
- diminished sexual desire,
all of which sabotage the efficacy of PDE5 inhibitors.
In the case described, testosterone levels below 220 ng/dL placed the patient squarely in the hypogonadal range, making sildenafil pharmacologically disadvantaged from the outset. This biological mismatch explains why he achieved no more than 60% erection quality even when using the medication correctly.
Therapeutic Course: Why Testosterone Supplementation Restored Sildenafil’s Effectiveness
The pivotal moment in the clinical narrative arrives with the introduction of transdermal testosterone gel. Within two weeks of therapy, the patient’s testosterone levels rose to mid-normal range (448 ng/dL total, 78 pg/mL free). His libido improved substantially—confirmed by both patient and spouse—and his sexual function scores increased. This improvement alone denotes the hormone’s centrality to sexual wellbeing.
However, the true transformation occurred when sildenafil was reintroduced. After testosterone normalization, sildenafil produced an erection response that the patient and his wife described as satisfactory—finally enabling reliable sexual activity. Objective measures confirmed the improvement: his erectile function domain score increased from 20 to 24, approaching the upper limits of normal.
This sequence demonstrates the synergistic relationship between testosterone and sildenafil. Testosterone corrects the upstream deficiencies—restoring NOS expression, enhancing NO availability, modulating PDE5 levels—and sildenafil amplifies the NO–cGMP pathway to its full potential. Neither therapy alone would have achieved the same result in this patient.
Critically, the success persisted six months after initiating testosterone therapy. The sustained benefit reinforces that the treatment addressed fundamental physiological deficits rather than providing a temporary enhancement.
Comparative Evidence: What Clinical Studies Reveal About Testosterone–PDE5 Inhibitor Synergy
Beyond the case study, broader clinical evidence substantiates the therapeutic pattern observed here. Guay and colleagues demonstrated that androgen supplementation improved sildenafil responsiveness in a cohort of hypogonadal men who previously failed monotherapy. Similarly, Shabsigh et al. showed that adding testosterone gel significantly improved erectile function within four weeks—a rapid therapeutic onset—and enhanced orgasmic function and overall satisfaction.
Several key takeaways emerge from these studies:
- Testosterone supplementation increases the percentage of responders to sildenafil.
- Improved libido contributes meaningfully to overall sexual satisfaction.
- The magnitude of improvement often plateaus at weeks 8–12, suggesting early responsiveness is the most clinically meaningful stage.
- Testosterone’s effect appears most pronounced in men with total levels below 300–350 ng/dL, reinforcing a threshold-based response.
Together, these data validate a clinical principle: testosterone supplementation should be strongly considered in hypogonadal men who fail initial PDE5 inhibitor therapy.
More Than Sexual Function: Broader Health Considerations in the Hypogonadal ED Patient
Management of ED in hypogonadal men extends beyond sexual function. Hypogonadism is associated with decreased bone density, reduced muscle mass, depressive symptoms, metabolic derangements, and reduced quality of life. Recognizing this, the case study clinician appropriately recommended bone densitometry—a step often forgotten in standard erectile dysfunction workups. This approach underscores that testosterone replacement is a holistic treatment, not simply a sexual enhancement strategy.
The vascular abnormalities observed on penile Doppler also warranted a cardiology referral. Erectile dysfunction can precede coronary artery disease by several years; thus, arterial insufficiency in the penis may be a surrogate marker for systemic endothelial dysfunction. Hypogonadal men are at increased cardiovascular risk—and appropriate screening can be lifesaving.
These broader implications highlight why ED evaluation should be considered an opportunity for comprehensive male health assessment rather than a narrowly focused intervention.
Clinical Lessons: How to Manage ED in Hypogonadal Men More Effectively
The therapeutic insights from this case are applicable across clinical practice. Successful management rests on a simple, structured sequence:
- Evaluate testosterone early.
Every man presenting with ED should have an early morning serum total testosterone level measured. This prevents years of ineffective PDE5 inhibitor trials. - Ensure proper sildenafil use.
The medication must be taken on an empty stomach, 30–60 minutes before sexual activity, and attempted on multiple occasions before declaring failure. - Reassess hormonal and vascular contributors.
Repeat testosterone measurement, free testosterone, LH, and prolactin levels help determine underlying pathology. - Introduce testosterone supplementation when clinically indicated.
Transdermal gel offers steady-state physiologic levels and high patient adherence. - Rechallenge PDE5 inhibitors after hormonal correction.
Once testosterone is normalized, PDE5 inhibitors often regain efficacy.
When applied together, these steps transform many cases of “sildenafil resistance” into successful long-term treatment outcomes.
Conclusion
The case of sildenafil failure in a hypogonadal man provides a profound demonstration of the interplay between endocrine balance and erectile physiology. Testosterone is not merely a libido regulator; it is a molecular architect of penile vascular function, nitric oxide signaling, and PDE5 responsiveness. Treating ED with sildenafil alone while ignoring hypogonadism is akin to applying a fresh coat of paint to a structurally compromised foundation—temporarily pleasing, but fundamentally ineffective.
By identifying androgen deficiency, correcting it with physiologic testosterone replacement, and subsequently reintroducing sildenafil, clinicians can restore erectile function, revive libido, and improve quality of life for men who might otherwise be labeled “nonresponders.” The biological logic is sound, the clinical evidence substantial, and the therapeutic outcome deeply satisfying.
FAQ
1. Why do hypogonadal men often fail to respond to sildenafil?
Because sildenafil requires nitric oxide to initiate cGMP signaling. Low testosterone reduces nitric oxide synthase expression, diminishing NO availability and compromising PDE5 inhibitor efficacy.
2. How quickly does testosterone supplementation improve erectile function?
Libido improvements often appear within 2–4 weeks. Sildenafil responsiveness may improve shortly thereafter, once testosterone reaches physiologic levels.
3. Should testosterone be given to all men who fail PDE5 inhibitors?
No. Only men with confirmed low testosterone levels should receive supplementation. Proper diagnostics—including repeat testosterone measurement—are essential before initiating therapy.