The early stages of human pregnancy are an intricate biological negotiation between embryo and endometrium. For implantation to succeed, the developing trophoblast must transition from a relatively sedentary epithelial identity into a highly motile, invasive subtype capable of remodeling maternal spiral arteries. This transformation is fundamental for establishing adequate uteroplacental blood flow and, by extension, for supporting fetal development. Yet this process is far from guaranteed; subtle disruptions can initiate a cascade of pathological events culminating in implantation failure, intrauterine growth restriction (IUGR), and pre-eclampsia—conditions that continue to challenge obstetric care worldwide.
Recent experimental work has brought an unlikely pharmacological candidate into the spotlight: sildenafil citrate, best known for its vasodilatory prowess in treating erectile dysfunction. At first glance, linking a PDE5 inhibitor with placental development might seem unconventional, if not slightly humorous. But science often rewards those who consider unusual connections. As it turns out, sildenafil interacts directly with core molecular pathways governing trophoblast differentiation and invasion. The study on which this article is based demonstrates, with elegant precision, that sildenafil activates nitric oxide (NO)–driven cyclic guanosine monophosphate (cGMP) signaling, triggering a phenotypic shift that transforms cytotrophoblasts into highly invasive, extravillous counterparts.
In other words, sildenafil may do for trophoblasts what it does for vascular smooth muscle: enhance relaxation, signaling, and movement—albeit with very different consequences.
Biological Context: Why Trophoblast Invasion Matters More Than We Admit
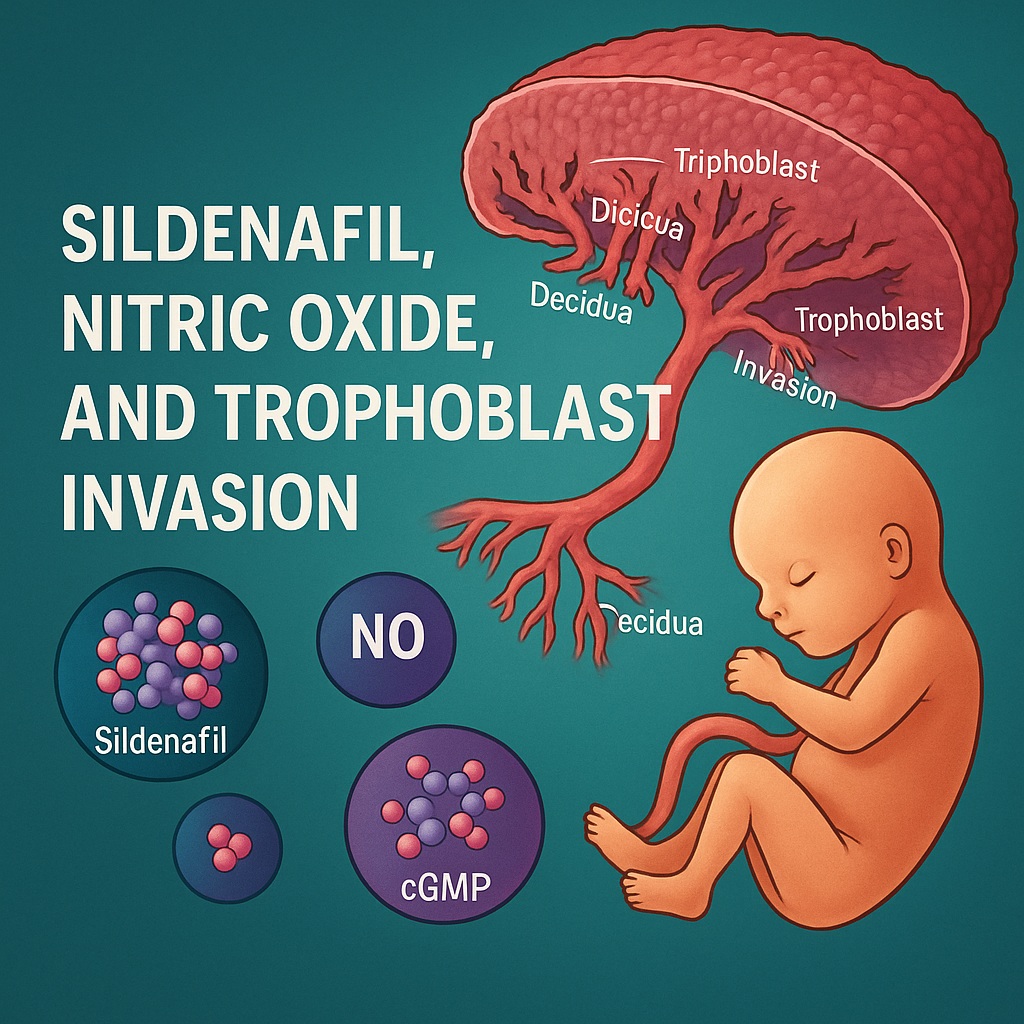
The development of the placenta represents one of the most sophisticated and intimate interactions between human tissues. Cytotrophoblasts at the maternal-fetal interface undergo a remarkable transition: they shed epithelial characteristics, adopt a mesenchymal phenotype, express new sets of integrins, and begin migrating into the decidua. The ultimate goal is remodeling spiral arteries into high-capacitance, low-resistance vessels that ensure sufficient blood flow to the fetus.
A few key principles govern this process. First, extravillous trophoblasts (EVTs) cannot remain static; they must be highly motile. Second, the shift from an epithelial integrin profile (dominated by α6β4) to a mesenchymal or invasive profile (α1β1) is essential for successful migration. And third, this transformation requires sensitive regulation by NO and cGMP—signaling molecules renowned for their roles in vascular physiology but equally critical in placentation.
When these mechanisms falter, the consequences are not subtle. Poor trophoblast invasion is linked with:
- Pre-eclampsia, characterized by endothelial dysfunction, impaired spiral artery remodeling, and excessive release of soluble antiangiogenic factors.
- IUGR, in which inadequate placental perfusion limits fetal development.
- Recurrent implantation failure, a frustrating clinical barrier in reproductive medicine.
Given these connections, enhancing trophoblast invasion is more than a biological curiosity; it represents a therapeutic frontier. This is precisely where sildenafil enters the discussion, bringing with it the capacity to influence NO–cGMP signaling with surprising effectiveness.
Study Framework: How Researchers Demonstrated Sildenafil’s Direct Role in Trophoblast Biology
The foundational investigation used two primary experimental systems: first-trimester chorionic villous explants and a well-established cytotrophoblast cell line (HTR-8/SVneo). These models allow careful examination of trophoblast outgrowth, invasion through extracellular matrices, and molecular markers of differentiation.
Researchers treated cells with sildenafil across a range of concentrations and also employed:
- A cGMP analog to mimic downstream signaling,
- A cGMP inhibitor to block its effects,
- The NO donor SNAP, and
- The NO synthase inhibitor L-NAME, to evaluate dependency on NO.
This comprehensive design allowed the team to isolate specific points in the signaling pathway and establish causal relationships rather than mere correlations.
Notably, all tissues were collected ethically following informed consent, and explant assays were meticulously repeated to validate reproducibility—a point often glossed over in field summaries but essential for interpreting the robustness of findings.
What emerged from this experimental framework is a compelling demonstration that sildenafil does far more than transiently increase perfusion. It directly rewires trophoblast phenotype and behavior through NO-dependent cGMP accumulation.
Sildenafil Enhances Trophoblast Outgrowth: Migration, Not Proliferation, Drives the Effect
One of the most striking findings arises from first-trimester villous explant cultures. When trophoblasts were exposed to sildenafil (350 ng/mL), the outgrowth from villous tips increased dramatically—more than fourfold compared to untreated controls. Bright-field microscopy revealed broad, dense outgrowths, replacing the sparse, thin projections typically seen in controls.
Importantly, the study confirmed that this impressive extension was not due to increased cell proliferation. Expression of Ki67, a nuclear proliferation marker, remained unchanged between treated and untreated groups. Cell counts in outgrowth regions were also comparable.
This distinction matters. Increasing trophoblast numbers without corresponding functional change would offer limited therapeutic insight. Instead, sildenafil’s effects center on enhanced motility, promoting the outward migration required for deep placentation. This finding echoes physiological observations where NO-derived cGMP regulates cytoskeletal dynamics, adhesion turnover, and directional movement.
The separation between proliferation and migration makes the trophoblast response more controlled and less likely to pose concerns regarding unchecked cellular expansion—a comforting feature for any potential therapeutic application.
Matrigel Invasion Assays Reveal Sildenafil’s Dose-Dependent Stimulation of Trophoblast Motility
To quantify invasive capacity more precisely, researchers used Matrigel-coated transwell chambers. These assays simulate the extracellular matrix environment that EVTs must traverse during implantation.
At concentrations of 350–3,500 ng/mL, sildenafil significantly increased invasion—up to four- to fivefold compared to vehicle controls. This magnitude of enhancement mirrors the degree of invasion induced by known cGMP analogs, reinforcing the idea that sildenafil’s mechanistic action hinges on intracellular cyclic nucleotide accumulation.
This dose-dependency suggests that trophoblasts respond to sildenafil in a controlled, predictable manner, rather than through a blunt overactivation of migration pathways. It also underscores that physiologically relevant concentrations produce measurable biological effects—an essential consideration for clinical translation.
Phenotypic Transformation: Integrin Switching as a Hallmark of Extravillous Differentiation
Perhaps the most elegant evidence of sildenafil’s influence stems from integrin profiling. Under baseline conditions, cytotrophoblasts express high levels of α6 (indicative of epithelial identity) and minimal α1 (associated with invasive capacity). After sildenafil treatment, particularly at higher concentrations, the expression pattern inverted: α6 decreased, α1 rose sharply, and a clear integrin switch emerged.
This transformation recapitulates the integrin transition essential for EVT formation. It signifies a shift from basement membrane adherence to interstitial migration—a prerequisite for arterial remodeling.
Integrin switching is not a superficial marker; it is a functional commitment. Changes in integrin repertoire recalibrate ligand-binding specificity, cytoskeletal coupling, and downstream signaling pathways that coordinate invasion. The ability of sildenafil to induce this transformation places it squarely within the domain of differentiation modulators rather than mere motility enhancers.
Moreover, the fact that integrin switching parallels the effects observed when cytotrophoblasts are cultured on Matrigel alone underscores that sildenafil activates molecular programs already intrinsic to trophoblast biology.
Mechanistic Core: NO and cGMP as the Signaling Axis Driving Sildenafil-Induced Invasion
To unravel the mechanism, researchers tested whether sildenafil’s effects persisted when critical components of the NO–cGMP pathway were blocked. The answer was unequivocal: without NO or cGMP, sildenafil lost its ability to induce integrin switching and invasion.
Three central findings drive this conclusion:
- NO donors (e.g., SNAP) mimicked sildenafil’s effects by increasing invasion and promoting integrin switching.
- cGMP analogs independently triggered trophoblast differentiation, demonstrating cGMP as the relevant downstream effector.
- L-NAME (NO synthase inhibitor) and a specific cGMP inhibitor independently abolished sildenafil-induced invasion.
Together, these results clarify that sildenafil does not generate cGMP on its own. It prevents cGMP degradation by inhibiting PDE5, but adequate NO must first activate guanylyl cyclase to synthesize cGMP. Without this upstream signal, the pathway remains dormant.
This mechanistic nuance explains why sildenafil can amplify trophoblast invasion only in tissues capable of producing or responding to NO. It also aligns with clinical observations: disorders characterized by impaired NO synthesis—such as pre-eclampsia—are precisely those where trophoblast invasion fails.
The study thus positions sildenafil not as a primary activator of invasive differentiation, but as a potent sensitizer that magnifies endogenous NO signaling.
Clinical Implications: Reframing Sildenafil as a Potential Modulator of Placentation
If we accept that excessive trophoblast quiescence contributes to serious pregnancy complications, and that sildenafil enhances trophoblast differentiation and invasion through a physiologically relevant mechanism, the clinical implications become difficult to ignore.
Pre-eclampsia, IUGR, and implantation failure share a unifying pathology: shallow trophoblast invasion and defective spiral artery transformation. By restoring NO–cGMP signaling and promoting integrin switching, sildenafil may support:
- Improved placental perfusion,
- Enhanced endovascular invasion,
- Better fetal nutrient delivery,
- Reduced trophoblast apoptosis during oxidative stress.
While enthusiasm must be tempered by the need for rigorous clinical trials, sildenafil’s mechanism aligns closely with known deficiencies in these disorders. In particular, patients with thin endometrium or recurrent implantation failure have shown promising responses to sildenafil in preliminary studies, which may reflect improved uterine receptivity and trophoblast function.
However, timing is crucial. Once placental endothelial dysfunction is advanced—as in late pre-eclampsia—interventions targeting trophoblast biology may be too late. Early application, ideally before pathological spiral artery remodeling is completed, is likely necessary for meaningful benefit.
This perspective invites clinicians to reconsider sildenafil not as an unconventional choice but as a targeted therapeutic aligned with the pathophysiology of placental insufficiency.
Current Limitations and Future Directions in Translational Applications
Despite compelling biological findings, several limitations must be acknowledged. The study relies heavily on in vitro and ex vivo models, which cannot capture the full complexity of uteroplacental hemodynamics. Trophoblast behavior is influenced by immune cells, endocrine cues, extracellular matrix architecture, and mechanical forces—all of which are simplified in controlled laboratory environments.
Additionally, the relationship between enhanced invasion and clinical safety must be carefully evaluated. While inadequate invasion is harmful, excessive invasion is equally problematic, as it may predispose to conditions like placenta accreta or percreta. Fortunately, current evidence suggests sildenafil amplifies physiological invasion rather than driving aberrant overinvasion, but the boundary requires careful study.
Further research should focus on:
- Longitudinal in vivo studies evaluating placental development,
- Dose-response and safety assessments during early gestation,
- Identification of biomarkers predicting treatment responsiveness,
- Integration of sildenafil with other modulators of uterine receptivity,
- Exploration of combination therapy with NO donors or L-arginine.
The NO–cGMP axis represents fertile ground for therapeutics. Sildenafil may be the first well-studied agent in this arena, but it is unlikely to be the last.
Conclusion
The study of sildenafil’s effects on human trophoblasts illuminates a previously underappreciated dimension of placental biology. By operating through the NO–cGMP signaling pathway, sildenafil promotes integrin switching, accelerates migration, and enhances invasion—features central to successful early placentation. These actions extend far beyond its traditional vascular applications and underscore the interconnectedness of endothelial and trophoblastic physiology.
With its capacity to counteract shallow trophoblast invasion, sildenafil emerges as a promising candidate for addressing implantation failure, IUGR, and pre-eclampsia—provided it is used early and judiciously. While more clinical data are needed before routine adoption, the mechanistic clarity provided by this study offers a compelling foundation for future research.
FAQ
1. Does sildenafil directly increase trophoblast proliferation?
No. Studies show no change in Ki67 expression or cell number in treated explants, indicating that sildenafil enhances migration and invasion, not proliferation.
2. Why is integrin switching important for implantation?
Integrin switching from α6β4 to α1β1 reflects the transformation from an epithelial to an invasive phenotype. This transition enables trophoblasts to remodel spiral arteries and is essential for normal placentation.
3. Can sildenafil be used clinically to prevent pre-eclampsia or IUGR?
Preliminary mechanistic data are promising, but clinical results remain mixed. Timing and patient selection appear critical. Large, controlled trials are necessary to determine safety and efficacy.