Pulmonary hypertension (PH) has gradually shifted from being a peripheral concern in β-thalassemia care to one of its central and most dangerous complications. For clinicians and researchers, the real question is no longer whether PH occurs in transfusion-dependent hemoglobinopathies—it clearly does—but what we can do to stop it early, safely, and effectively. A recent randomized clinical trial evaluating L-arginine and sildenafil in β-thalassemia children with increased tricuspid regurgitant jet velocity (TRJV) offers an opportunity to examine this issue in depth. Beneath the statistically neat tables lies a vivid story about vascular biology, nitric oxide deprivation, hemolysis, oxidative stress, and the delicate interplay of pediatric cardiopulmonary physiology.
This article dissects the core findings of the study and places them in the broader context of modern thalassemia management, translating the research into a comprehensive analysis suitable for practitioners, hematologists, pediatricians, and academics. The tone is intentionally clear and professional, avoiding the fog of overly academic language while retaining scientific rigor. Where appropriate, we allow ourselves a subtle note of irony—after all, few conditions manage to involve hemolysis, endothelial dysfunction, iron overload, hypercoagulability, and nitric-oxide scavenging quite as simultaneously as β-thalassemia does.
Understanding the Vascular Burden of β-Thalassemia
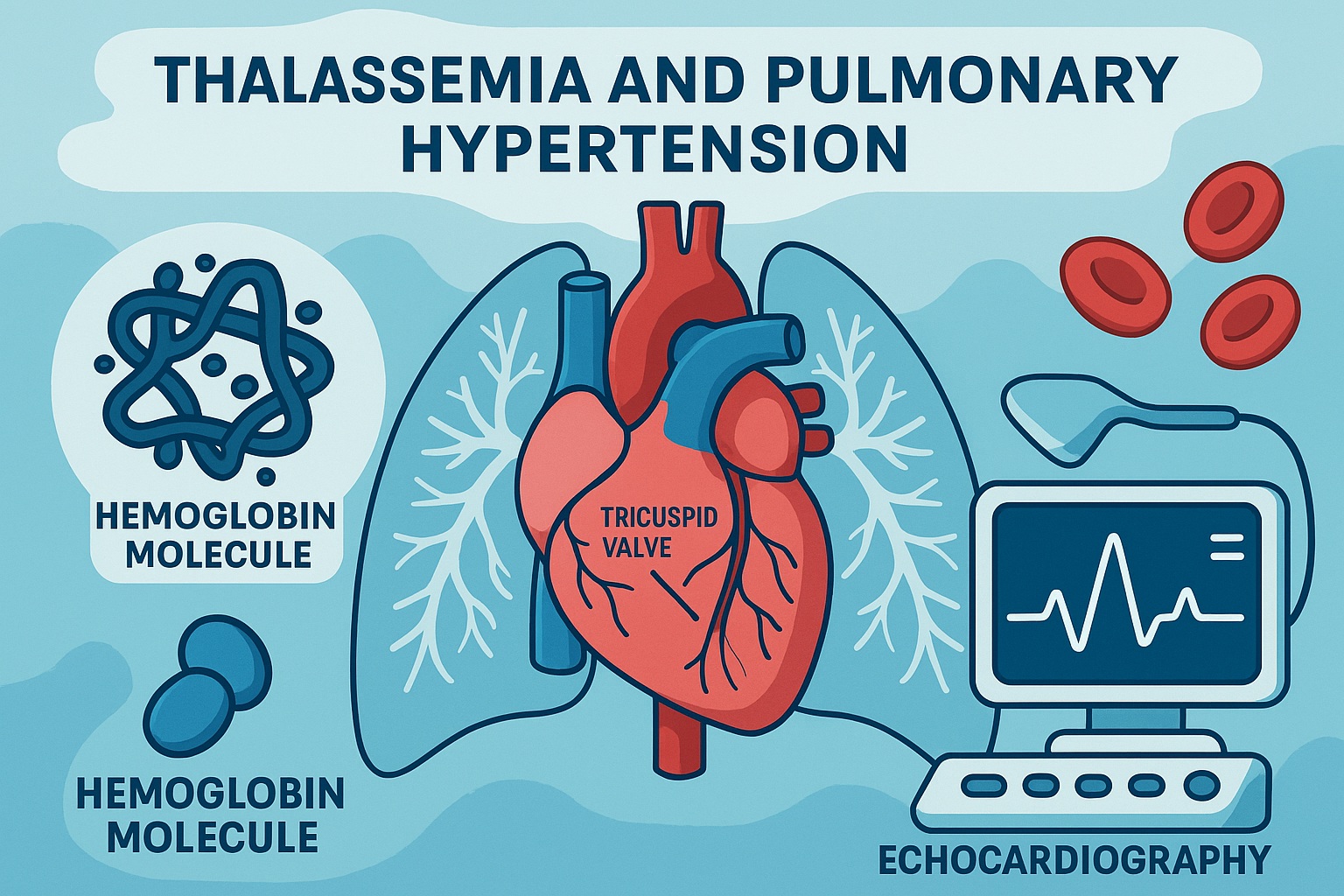
The pathophysiology of PH in β-thalassemia is anything but simple, yet certain mechanistic pillars reliably support its progression. First, chronic hemolysis destroys red cells at a pace that floods the circulation with cell-free hemoglobin and arginase, both of which sabotage nitric oxide (NO) availability. NO, the quiet custodian of vasodilation and endothelial balance, becomes rapidly consumed or blocked. Without it, pulmonary vascular tone increases and the smooth muscle layers of the pulmonary arteries begin to thicken, remodel, and constrict.
Children with β-thalassemia major, especially those dependent on transfusions, face an additional burden: iron overload. Excess iron is a catalytic force for oxidative stress, damaging tissues and further impairing endothelial function. Even the heart is not spared—iron deposition in myocardial cells contributes to diastolic dysfunction, a subtle but potent precursor to pulmonary congestion and elevated pulmonary pressures.
Another overlooked culprit is platelet activation. Without adequate NO to restrain it, the coagulation system becomes hyperreactive. Activated platelets initiate microthrombi formation, particularly in the pulmonary vasculature, where blood flow dynamics already teeter on the edge of pathology. Over years, these microthrombi narrow vascular channels and raise pulmonary arterial pressure—quietly, progressively, and often asymptomatically.
TRJV, easily measured on Doppler echocardiography, becomes a valuable early biomarker of this dysfunction. A value above 2.5 m/s should raise suspicion, while higher values point toward developing PH. In children—in whom symptoms may be masked by excellent physiological compensation—measurement is crucial.
Here lies the rationale for therapeutic exploration: if the NO pathway is disrupted, can it be replenished? If the pulmonary vasculature is overly constricted, can we relax it? The study in question investigates exactly these possibilities using L-arginine, the substrate for NO synthesis, and sildenafil, a phosphodiesterase-5 inhibitor already familiar to cardiologists.
Why L-Arginine and Sildenafil? Two Treatments, One Pathway
L-arginine and sildenafil address the same pathway from different angles. Understanding this duality is essential for appreciating the study’s purpose and outcomes.
L-arginine is the biochemical starting point for NO production. In a child with β-thalassemia, arginase released from destroyed red cells usually diverts arginine toward ornithine and polyamine synthesis rather than NO synthesis. The hypothesis is simple: provide enough L-arginine, and perhaps there is enough substrate to overcome the metabolic detour and restore NO levels. Theoretically, improved NO levels should relax pulmonary vessels, reduce endothelial activation, and decrease TRJV.
Sildenafil, meanwhile, does not create NO—but it protects what remains of its effects. By inhibiting PDE5, sildenafil prevents the breakdown of cyclic GMP (cGMP), the intracellular messenger responsible for mediating NO-induced vasodilation. In practical terms, sildenafil prolongs vasodilation in pulmonary arteries, reduces pulmonary vascular resistance, and improves right-ventricular afterload. Given the drug’s established use in other forms of pulmonary hypertension, its application in hemolytic anemias is a logical extension.
The study tests both approaches head-to-head in pediatric β-thalassemia major patients with elevated TRJV, seeking to determine not only whether they work, but how they compare to each other.
Clinical Trial Overview: Design, Methodology, and Population
The randomized controlled trial enrolled 60 children with β-thalassemia major who met a strict criterion of TRJV greater than 2.5 m/s. All participants were between 6 and 18 years old and were drawn from the pediatric hematology unit of a major university hospital. This group represents a typical high-risk thalassemia population: transfusion-dependent, exposed to iron overload, and vulnerable to the cardiovascular complications inherent to chronic hemolysis.
Participants were divided into three matched groups:
• Group I – L-arginine therapy (0.1–0.2 g/kg/day for one month)
• Group II – Sildenafil therapy (0.25 mg/kg/dose every 6 hours for one month)
• Group III – Control group, receiving neither intervention
All children underwent baseline and post-treatment evaluations, including:
- complete blood count
- liver function tests
- serum ferritin
- Doppler echocardiography to reassess TRJV
Notably, no significant differences were found among groups at baseline in terms of hemoglobin levels, liver enzymes, ferritin, or TRJV. This offers essential reassurance that any treatment effect is likely real and not merely the result of group differences.
The Study Results: When Physiology Meets Pharmacology
While the laboratory parameters remained largely unchanged across all groups, the cardiovascular outcomes tell a compelling story. After one month of therapy, both intervention groups—L-arginine and sildenafil—showed a significant reduction in TRJV. In clinical practice, a decline in TRJV reflects reduced pulmonary arterial pressure and improved right-ventricular loading conditions, both highly desirable outcomes.
Sildenafil demonstrated the slightly stronger numerical improvement, although the study did not find a statistically significant difference between the two active treatments. In both cases, TRJV reductions were meaningful, while the control group predictably showed no improvement at all. The study thereby reinforces the notion that PH in β-thalassemia is modifiable, at least in its early stages.
It is especially notable that this improvement occurred over a relatively short treatment period of just one month—a testament to how responsive pulmonary vessels can be when NO signaling is effectively supported or amplified.
These findings strongly support incorporating targeted vasodilatory therapy into early PH management protocols for pediatric β-thalassemia. And although larger and longer studies are needed, real-world clinical experience aligns with these results: children respond, symptoms improve, and echocardiographic markers shift favorably.
Interpreting the Findings Through a Clinical Lens
For pediatric hematologists, interpreting the application of these findings requires a balance of enthusiasm and caution. On one hand, the pathophysiological logic behind both treatments is robust. Hemolysis impairs the NO pathway; both L-arginine and sildenafil bolster it. The study’s finding—that TRJV improves even over four short weeks—validates this understanding.
On the other hand, pulmonary hypertension in β-thalassemia is rarely a short-term problem. It arises from cumulative years of hemolysis, iron overload, organ dysfunction, and chronic oxidative stress. No one expects a single month of therapy to erase all vascular remodeling. What the study reveals instead is that the pulmonary vasculature of thalassemic children retains a significant degree of reversibility when targeted early and strategically.
This raises compelling clinical considerations:
Early monitoring is essential.
Relying on symptoms is unreliable because children compensate well. Regular TRJV assessment during routine echocardiography may reveal problems long before symptoms appear.
Combination therapy may be the future.
While not explored in this study, concurrent arginine supplementation and PDE5 inhibition may theoretically address NO deficiency more powerfully.
Iron overload must be controlled.
Chelation remains the backbone of prevention, as iron catalyzes the oxidative processes that worsen endothelial dysfunction.
Hypercoagulability cannot be ignored.
Sildenafil’s additional benefit in reducing platelet activation is particularly valuable in thalassemic physiology.
Ultimately, the real lesson of the study is not that sildenafil or L-arginine alone will cure PH in β-thalassemia, but that the pulmonary disease process is treatable—and perhaps preventable—if clinicians intervene early and consistently.
Broader Implications for Thalassemia Management
Pulmonary hypertension has historically been overshadowed by cardiac iron overload and endocrine complications in thalassemia care. Yet the more closely we examine it, the clearer it becomes that PH is not a rare complication at all. It lurks quietly behind years of transfusions, high cardiac output, splenectomy, and persistent hemolysis.
The ability of simple pharmacological interventions such as sildenafil and L-arginine to create measurable improvements underscores an important shift in clinical thinking:
thalassemia care must look beyond hemoglobin levels and ferritin readings to include vascular health as a routine focus.
This shift has several practical implications for modern clinical practice:
- Echocardiographic surveillance should be standardized across pediatric thalassemia programs.
- L-arginine, as an inexpensive, safe supplement, may serve as a first-line strategy for borderline cases of elevated TRJV.
- Sildenafil may be introduced for more significant elevations or for patients who show insufficient response to other measures.
- Multidisciplinary collaboration between hematologists, cardiologists, and pulmonologists can dramatically improve patient outcomes.
Perhaps most importantly, this study reminds clinicians that physiological pathways disrupted in complex genetic diseases can sometimes be restored with surprising ease—if one understands where the bottleneck lies.
Conclusion
The comparative analysis of L-arginine and sildenafil in β-thalassemia children with elevated TRJV illustrates an encouraging truth: even in a disease dominated by chronic hemolysis, vascular dysfunction, and iron overload, the pulmonary vasculature remains responsive to treatment. Both L-arginine and sildenafil significantly reduced TRJV, offering a measurable improvement in a key marker of pulmonary hypertension.
The study contributes meaningfully to the growing body of literature highlighting the role of NO deficiency in thalassemia-associated pulmonary vascular disease and reinforces the therapeutic relevance of restoring or amplifying NO-dependent pathways.
While further research is needed to determine long-term efficacy, optimal dosing, and combined therapeutic strategies, the findings provide clinicians with actionable options today. In an area of pediatric hematology where early intervention can alter the trajectory of a child’s life, such insights are invaluable.
FAQ
1. Is sildenafil safe for children with β-thalassemia and elevated TRJV?
Yes. Clinical studies, including the one analyzed here, indicate that sildenafil is well tolerated in pediatric populations when dosed appropriately. It has a well-established safety profile in various forms of pulmonary hypertension.
2. Can L-arginine be used long-term to prevent pulmonary hypertension in thalassemia?
While L-arginine is generally safe and physiologically rational, long-term preventive data are limited. It may be beneficial for early endothelial dysfunction, but regular clinical monitoring is still necessary.
3. Does lowering TRJV guarantee prevention of pulmonary hypertension?
Not entirely. TRJV is a screening indicator, not a definitive diagnostic measure. However, reducing TRJV reflects improved pulmonary hemodynamics and is associated with better clinical outcomes. Combining this with optimal transfusion and chelation therapy yields the best preventive strategy.