Introduction
Few drugs in the modern pharmacological lexicon have experienced as dramatic a transformation in reputation as sildenafil. Once dismissed as a failed antihypertensive, it has become a global icon of sexual medicine. Yet behind this well-known story lies a far richer and more intriguing pharmacological narrative—one that reaches far beyond erectile dysfunction (ED).
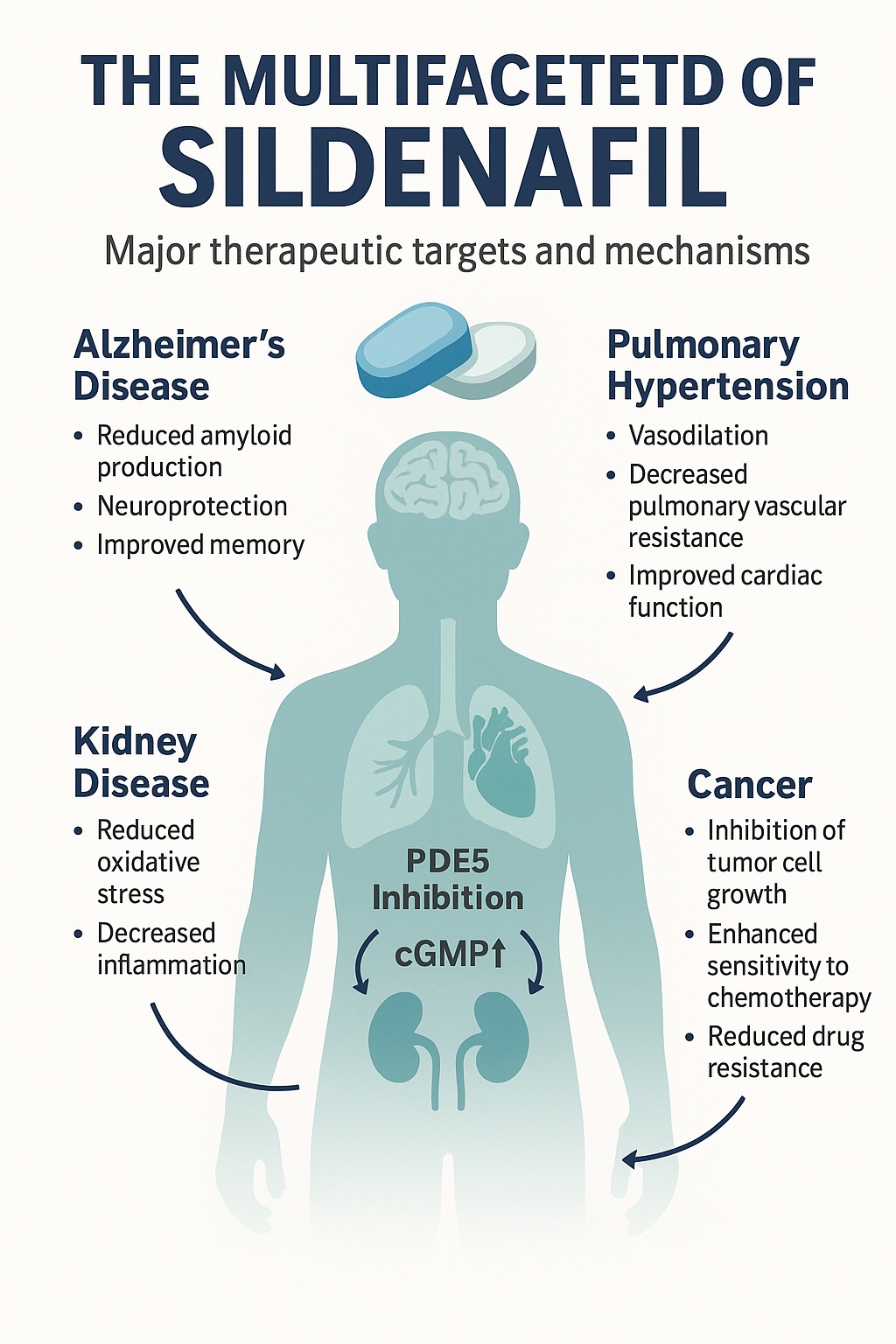
Sildenafil’s mechanism—selective inhibition of phosphodiesterase type 5 (PDE5)—has revealed a cascade of effects throughout multiple organ systems. By increasing intracellular cyclic guanosine monophosphate (cGMP) and enhancing nitric oxide (NO) signaling, sildenafil influences vascular tone, cellular metabolism, and even neuronal resilience. This pharmacological breadth has sparked growing interest in sildenafil as a multi-disease modulator—a single molecule capable of therapeutic relevance across cardiovascular, neurological, renal, and oncologic domains.
This article delves into the multifaceted medical potential of sildenafil, examining how a once narrowly defined drug has emerged as a versatile tool in modern therapeutics.
Mechanistic Foundations: PDE5, cGMP, and the NO Pathway
At its core, sildenafil targets a fundamental biological regulator—PDE5, an enzyme responsible for cGMP degradation. Under normal physiology, cGMP mediates smooth muscle relaxation through the NO–cGMP–protein kinase G (PKG) cascade. When PDE5 breaks down cGMP into inactive GMP, vasodilation ceases. Inhibiting PDE5 therefore sustains cGMP levels, prolonging vascular smooth muscle relaxation and improving tissue perfusion.
What makes this pathway medically fascinating is its ubiquity. PDE5 is expressed in the pulmonary vasculature, corpus cavernosum, retina, brain, kidney, and even tumor tissues. Thus, sildenafil’s biochemical influence extends far beyond a single organ system.
Furthermore, cGMP signaling interacts with mitochondrial metabolism, calcium flux, and oxidative stress. Activation of PKG can stimulate mitochondrial biogenesis, reduce apoptosis, and modulate inflammatory cascades—mechanisms central to diseases as diverse as Alzheimer’s, ischemia, and chronic pain.
Pharmacokinetics and Safety: A Balanced Profile
Sildenafil is rapidly absorbed, reaching peak plasma concentration within one hour. It undergoes hepatic metabolism primarily via CYP3A4, producing an active metabolite, N-desmethyl sildenafil, with approximately half the potency of the parent drug. Its half-life ranges from 3 to 5 hours, with elimination mainly through feces.
The drug’s safety profile is well-established: mild headache, flushing, dyspepsia, and transient visual changes are the most common effects, all resulting from its vasodilatory action. However, the hemodynamic interaction with nitrates remains the principal contraindication, as combined vasodilation may cause life-threatening hypotension.
In clinical use, sildenafil’s tolerability and short duration make it an attractive candidate for repurposing across chronic diseases that require controlled vascular modulation without systemic toxicity.
Sildenafil in Neurology: From Memory to Neuroprotection
One of the most exciting research frontiers for sildenafil lies within the brain. Despite its peripheral pharmacological fame, sildenafil crosses the blood–brain barrier, influencing central PDE5 activity.
Alzheimer’s Disease
Neurodegeneration in Alzheimer’s involves mitochondrial dysfunction, oxidative stress, and neuroinflammation—all processes modulated by cGMP signaling. Sildenafil enhances PGC-1α expression, promoting mitochondrial biogenesis and antioxidant enzyme upregulation. Moreover, it reduces β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) expression, decreasing amyloid-beta accumulation—a hallmark of Alzheimer’s pathology.
In animal models, sildenafil improved memory, reduced neuroinflammation, and increased brain-derived neurotrophic factor (BDNF) levels. Early clinical studies have confirmed enhanced cerebral blood flow and oxygen metabolism, hinting at therapeutic potential beyond symptomatic relief.
Depression and Neuropsychiatric Disorders
Depression and erectile dysfunction often coexist, sharing common biochemical roots in NO and serotonin pathways. By enhancing cGMP signaling and modulating monoamine transmission, sildenafil has demonstrated antidepressant-like effects in animal models and improved mood in clinical studies of men with comorbid ED and depression.
This dual benefit—restoring both sexual and emotional vitality—illustrates how neurovascular and neurochemical modulation intersect in sildenafil’s pharmacology.
Vascular Medicine and Raynaud’s Phenomenon
Sildenafil’s vasodilatory action naturally extends to disorders of the microvasculature. Raynaud’s phenomenon and systemic sclerosis–related digital ulcers exemplify pathologies driven by endothelial dysfunction and reduced NO bioavailability.
Clinical trials have consistently shown that sildenafil reduces the frequency, severity, and duration of Raynaud’s attacks, improves digital blood flow, and accelerates ulcer healing. Its synergistic use with bosentan, an endothelin receptor antagonist, further enhances outcomes, particularly in systemic sclerosis.
These findings affirm sildenafil as not merely a vasodilator but as a microcirculatory stabilizer—a role of immense importance in chronic ischemic and autoimmune vascular diseases.
Wound Healing: Accelerating Repair Through cGMP
Wound repair is a symphony of vascular, inflammatory, and regenerative signals—many of which are tuned by NO and cGMP. Studies reveal that sildenafil accelerates angiogenesis, enhances collagen deposition, and reduces oxidative stress in animal models of cutaneous wounds.
Topical sildenafil formulations and hydrogels have shown remarkable potential in diabetic ulcer healing, improving neovascularization and tissue oxygenation. By maintaining endothelial viability and stimulating fibroblast proliferation, sildenafil may redefine chronic wound management, especially in ischemic or diabetic contexts.
Cancer: A Double-Edged Molecule with Adjuvant Potential
The discovery that PDE5 is overexpressed in multiple tumors—including prostate, breast, lung, and colorectal cancers—has transformed how oncologists view sildenafil. Elevated PDE5 levels are linked to tumor growth and resistance to apoptosis, making its inhibition a potential anticancer strategy.
Sildenafil enhances the cytotoxicity of chemotherapeutic agents like doxorubicin and cisplatin, promotes reactive oxygen species (ROS) generation, and suppresses anti-apoptotic proteins such as Bcl-xL. Furthermore, it blocks ABC drug efflux transporters (ABCC4, ABCB1, ABCG2), overcoming multidrug resistance and improving drug accumulation within tumors.
In animal models, combining sildenafil with chemotherapy significantly increased apoptosis and reduced tumor volume without additional toxicity. Clinical studies, though preliminary, suggest a promising role for sildenafil as a chemosensitizer and pharmacokinetic enhancer.
The Renal System: Protecting the Kidneys from Within
Chronic kidney disease (CKD) remains one of the most elusive therapeutic challenges. The renal endothelium, rich in PDE5, is central to filtration and microvascular regulation. Sildenafil’s capacity to restore NO–cGMP balance has shown renoprotective effects across a spectrum of kidney injuries, from diabetic nephropathy to ischemia–reperfusion injury.
Mechanistically, sildenafil:
- Activates endothelial and inducible NO synthases (eNOS/iNOS).
- Enhances mitochondrial biogenesis via PGC-1α.
- Reduces oxidative stress and inflammatory cytokines (TNF-α, IL-6).
- Regulates apoptotic pathways through the Bcl-2/Bax ratio.
Clinical data further confirm improvements in glomerular filtration rate, albuminuria, and creatinine clearance, supporting sildenafil’s candidacy as an adjunct therapy in renal dysfunction.
Pain Management: Redefining Analgesia
Pain, especially neuropathic pain, remains an area of unmet need. The NO–cGMP–PKG pathway influences nociceptive processing, and sildenafil’s ability to elevate cGMP within sensory neurons confers significant antinociceptive properties.
Experimental models demonstrate that sildenafil reduces thermal hyperalgesia and mechanical allodynia, likely through GABAergic modulation and potassium channel activation. Furthermore, by preserving angiopoietin-1 expression, sildenafil contributes to neural stability and vascular support, addressing both the vascular and neuronal dimensions of chronic pain.
Ophthalmology: Between Light and Vision
The eye provides both opportunity and caution for PDE5 inhibitors. While sildenafil’s mild inhibition of PDE6 explains transient visual disturbances, its broader retinal effects are beneficial in ischemic retinopathies.
By dilating choroidal vessels and reducing vascular endothelial growth factor (VEGF) levels, sildenafil mitigates retinal ischemia and neovascularization. Animal studies confirm decreased retinal thickness and oxidative stress markers, suggesting a potential role in diabetic and hypertensive retinopathy.
With careful dosing, sildenafil could emerge as a therapeutic adjunct in ocular ischemic disorders—an unexpected irony for a drug once blamed for blue-tinged vision.
The Cardiopulmonary Axis: Expanding on Known Territory
Pulmonary arterial hypertension (PAH) remains one of sildenafil’s established indications. Yet, research continues to reveal its broader cardiovascular utility. By reducing pulmonary vascular resistance and improving right ventricular function, sildenafil prolongs survival in PAH patients and enhances exercise tolerance.
Beyond PAH, sildenafil’s endothelial-restorative and anti-inflammatory properties may benefit heart failure, myocardial ischemia, and even post-surgical recovery. By improving endothelial NO availability and reducing fibrosis, sildenafil represents a bridge between vasodilation and vascular healing.
The Future of Drug Repurposing: Sildenafil as a Molecular Template
The renaissance of sildenafil in medical research reflects a broader paradigm—drug repurposing. Rather than abandoning old molecules, modern pharmacology reinterprets them through new mechanistic lenses. Sildenafil’s journey from angina to ED, and now to neuroprotection and oncology, exemplifies this philosophy.
The NO–cGMP–PKG signaling axis touches nearly every physiological system. Therefore, PDE5 inhibition represents not just symptomatic relief but a universal regulatory mechanism capable of recalibrating cellular homeostasis in disease states.
As research expands, it’s plausible that sildenafil—or its analogs—will evolve into multi-target therapeutics addressing chronic inflammatory, degenerative, and metabolic diseases simultaneously.
Conclusion
Sildenafil’s story is no longer a tale of serendipity—it is one of scientific reinvention. Once dismissed as a failed vasodilator, it now stands at the intersection of vascular biology, neurochemistry, and cellular metabolism. From restoring memory and healing wounds to sensitizing cancer cells and protecting the kidneys, sildenafil exemplifies the modern medical ideal: a molecule that adapts its purpose to meet evolving clinical needs.
In the years to come, sildenafil’s role may expand even further—not because it changes, but because medicine finally understands the depth of its design.
FAQ: The Multifaceted Potential of Sildenafil
1. Why does sildenafil have effects beyond erectile dysfunction?
Because PDE5 and cGMP signaling are present in multiple tissues—brain, kidney, lung, and even tumor cells—sildenafil’s inhibition of PDE5 influences various physiological processes, including inflammation, apoptosis, and vascular remodeling.
2. Can sildenafil really help in diseases like Alzheimer’s or cancer?
Preclinical evidence strongly suggests neuroprotective and chemosensitizing effects through cGMP–PKG activation, mitochondrial support, and modulation of apoptotic signaling. Clinical validation is ongoing, but early results are encouraging.
3. Is long-term sildenafil use safe outside sexual medicine?
At therapeutic doses, sildenafil’s adverse effects remain mild. However, careful monitoring is necessary for patients with cardiovascular disease, renal impairment, or those using nitrate medications due to potential hypotension.