Introduction
Two decades after its introduction, sildenafil citrate remains an iconic molecule in pharmacology — both for its revolutionary role in the treatment of erectile dysfunction (ED) and for its expanding applications in pulmonary arterial hypertension (PAH) and beyond. Originally developed as a vasodilator for coronary disease, sildenafil found fame through an unexpected side effect — improved penile hemodynamics — and has since become one of the most prescribed drugs in men’s health.
Yet, despite its familiarity, sildenafil’s safety profile continues to evolve. Controlled trials, while invaluable, cannot capture every nuance of drug behavior once it enters the real world — a world of comorbidities, polypharmacy, and variable adherence. That’s where pharmacovigilance comes in: a systematic, post-marketing surveillance mechanism for detecting adverse events (AEs) across large populations.
The study analyzed here, a real-world pharmacovigilance investigation using the U.S. FDA Adverse Event Reporting System (FAERS), offers a panoramic view of sildenafil’s safety experience. By exploring millions of AE reports over several years, the authors identified patterns, associations, and emerging safety signals that complement — and sometimes challenge — traditional clinical data. The findings shed light not only on sildenafil’s pharmacodynamics but also on how it behaves in complex clinical environments.
The Value of Pharmacovigilance in the Post-Marketing Era
Pharmacovigilance is more than a bureaucratic exercise; it is the epidemiology of drug safety. Through systems like FAERS, spontaneous reports from healthcare providers, manufacturers, and patients are aggregated into a database that can reveal previously undetected or underestimated risks.
This real-world dataset provides advantages over controlled clinical trials:
- It captures rare or delayed adverse events that might not surface in limited pre-approval studies.
- It reflects diverse patient populations, including the elderly and those with comorbid conditions.
- It allows for signal detection — statistical identification of disproportionate reporting frequencies compared to other drugs.
However, FAERS data have inherent limitations: underreporting, reporting bias, and lack of denominator data (the total number of drug exposures). Consequently, signal detection is hypothesis-generating rather than conclusive. The present study exemplifies this approach — combining quantitative disproportionality analysis with pharmacological interpretation.
Study Design: Mining the FAERS Database
The authors retrieved sildenafil-related adverse event data spanning multiple years from the FDA Adverse Event Reporting System, focusing on both branded and generic formulations. Each report includes variables such as patient demographics, reported adverse reactions (classified via MedDRA terms), reporting source, and outcome (e.g., hospitalization, death, disability).
Key methodological features included:
- Data Cleaning: Exclusion of duplicates and incomplete entries.
- Signal Detection Algorithms: Primarily the Reporting Odds Ratio (ROR) and Proportional Reporting Ratio (PRR), standard tools for assessing disproportionality.
- Stratification by Indication: Events associated with erectile dysfunction were analyzed separately from those for pulmonary hypertension, recognizing their distinct patient populations.
This rigorous framework allowed for differentiation between indication-driven risk profiles and those related to sildenafil’s pharmacologic class effects.
Overview of Reported Adverse Events
Unsurprisingly, the most frequently reported sildenafil-related adverse events involved cardiovascular, visual, and gastrointestinal systems. However, the relative prominence of these categories varied depending on the indication.
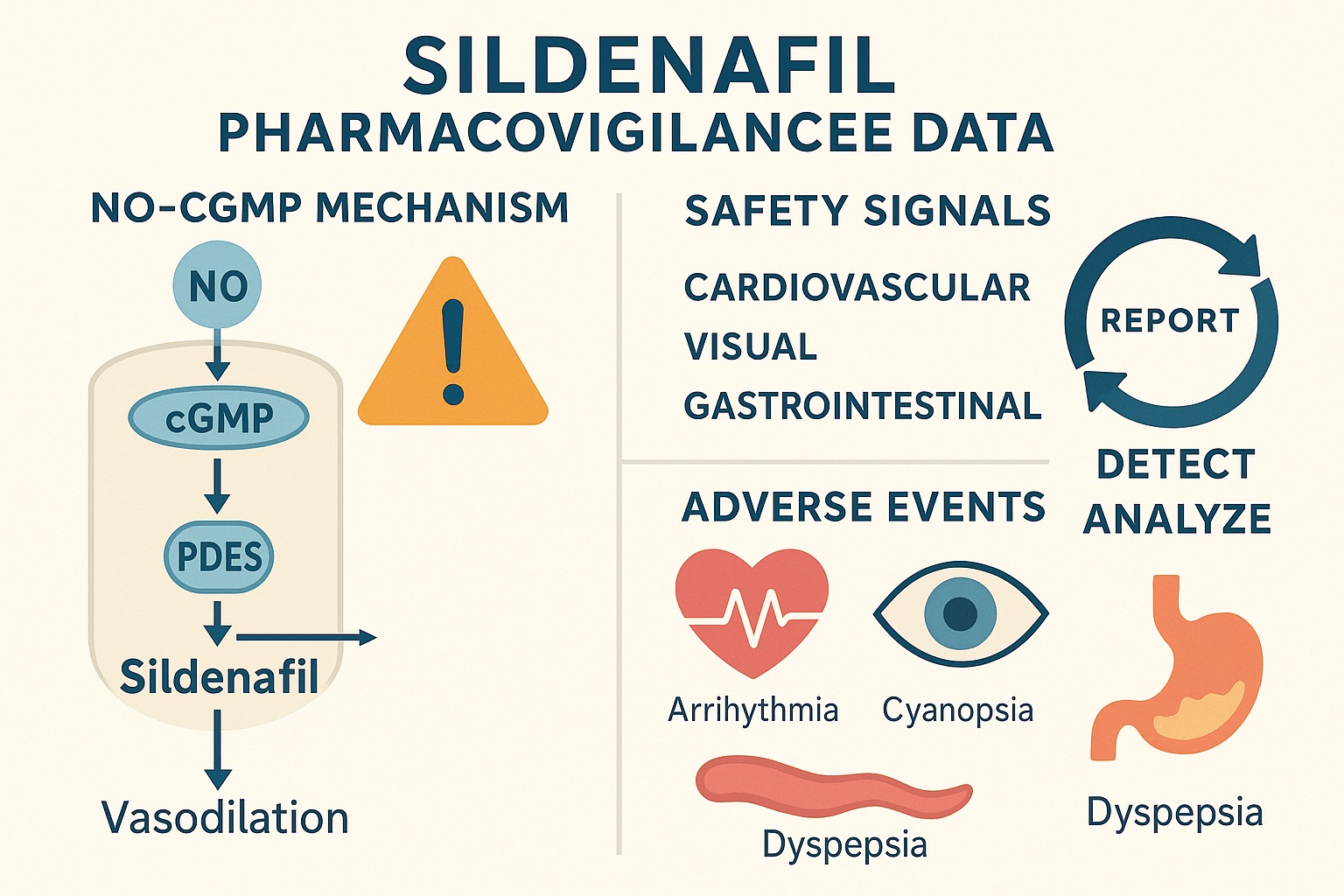
Cardiovascular Effects
Cardiovascular events dominated the dataset, consistent with sildenafil’s vasodilatory mechanism. Commonly reported events included hypotension, tachycardia, palpitations, chest discomfort, and syncope. Rare but serious cases of myocardial infarction and sudden cardiac death were also documented.
Importantly, the majority of cardiovascular events occurred in individuals with preexisting risk factors — hypertension, coronary artery disease, diabetes, or concomitant nitrate therapy. The pharmacological basis lies in sildenafil’s inhibition of PDE5 in vascular smooth muscle, which potentiates nitric oxide (NO)–mediated vasodilation and can synergize with nitrates to produce profound hypotension.
Visual and Neurological Disturbances
Another well-recognized signal involves sildenafil’s effect on visual perception. The FAERS data confirmed reports of color vision changes (cyanopsia), blurred vision, photophobia, and, in rare instances, non-arteritic anterior ischemic optic neuropathy (NAION).
The mechanism stems from cross-reactivity with PDE6, a phosphodiesterase subtype expressed in retinal photoreceptors. Although these visual effects are generally transient and dose-dependent, the persistence of NAION cases warrants caution, particularly in patients with vascular risk factors or optic disc anomalies.
Gastrointestinal and Musculoskeletal Complaints
Adverse events such as dyspepsia, nausea, flushing, and headache were also prevalent — predictable outcomes of systemic vasodilation and smooth muscle relaxation. Additionally, back pain and myalgia were sporadically reported, possibly reflecting PDE11 inhibition, though this remains speculative.
Emerging Safety Signals: Beyond the Expected
While many adverse events align with sildenafil’s established pharmacology, the FAERS analysis also revealed unusual or under-recognized patterns deserving further investigation.
Hearing Impairment
Cases of sudden sensorineural hearing loss (SSHL) associated with sildenafil surfaced as a notable signal. Although the incidence is low, the temporal relationship — often within hours of dosing — and the biological plausibility via cochlear NO-cGMP dysregulation support a possible causal link.
This aligns with prior case reports and FDA label updates warning of auditory disturbances. Clinicians should therefore inquire about hearing symptoms during long-term or high-dose use.
Psychiatric and Behavioral Effects
Interestingly, a subset of reports described anxiety, insomnia, agitation, and, rarely, impulsive sexual behavior. While causal inference remains uncertain, such effects may reflect central dopaminergic modulation secondary to enhanced NO signaling within the mesolimbic system — a pharmacological ripple that underscores sildenafil’s CNS penetration.
This neurobehavioral dimension, though subtle, invites further neuropharmacologic exploration, especially as PDE5 inhibitors gain attention for potential neurological and cognitive indications.
Drug Interactions
Polypharmacy emerged as a major driver of adverse outcomes. Reports involving nitrates, alpha-blockers, protease inhibitors (e.g., ritonavir), and antihypertensives were disproportionately represented. These combinations potentiate sildenafil’s vasodilatory and metabolic effects, amplifying systemic exposure and toxicity.
Such findings reinforce the necessity for careful medication reconciliation and patient education, particularly in elderly or multimorbid populations.
Demographic Trends: Who Is Reporting What?
Demographic stratification yielded telling insights:
- Male predominance (≈90%) reflected sildenafil’s primary indication in erectile dysfunction.
- Mean age skewed toward older adults, consistent with the epidemiology of both ED and cardiovascular comorbidities.
- Reports in women and pediatric populations, though rare, corresponded to sildenafil’s use in pulmonary hypertension, where the safety landscape differs markedly.
In the PAH subset, serious adverse events were more common, likely reflecting underlying disease severity and chronic dosing. Nonetheless, the overall benefit-risk ratio remained favorable when sildenafil was appropriately monitored.
Mortality and Serious Adverse Outcomes
Among serious outcomes, death, hospitalization, and life-threatening reactions accounted for a minority of reports but carried significant clinical implications.
Most fatalities were multifactorial, often involving cardiovascular collapse in patients with established ischemic heart disease. Importantly, no clear evidence emerged of direct sildenafil cardiotoxicity; rather, the drug appeared to unmask latent cardiovascular vulnerability under stress.
This underscores the value of comprehensive pre-prescription cardiovascular evaluation, especially in men with poorly controlled hypertension or angina. Sildenafil itself is rarely the villain — but it can expose preexisting fragility in the cardiovascular system.
Pharmacological Context: Mechanistic Insights
To interpret pharmacovigilance data responsibly, one must align it with pharmacodynamic principles. Sildenafil’s selective inhibition of PDE5 elevates intracellular cGMP, promoting smooth muscle relaxation and vasodilation.
Yet, PDE5 is not confined to penile vasculature. It is expressed in pulmonary arteries, systemic vasculature, platelets, and select CNS regions. Consequently, sildenafil’s systemic effects mirror the distribution of PDE5, explaining many off-target phenomena.
- Cardiovascular AEs stem from excessive vasodilation and reflex tachycardia.
- Visual AEs derive from PDE6 inhibition in the retina.
- Neurological effects may reflect secondary modulation of cyclic nucleotide signaling in the brain.
The study’s strength lies in confirming these theoretical mechanisms through empirical post-marketing evidence.
Comparative Analysis: Sildenafil vs. Other PDE5 Inhibitors
Contextualizing sildenafil’s safety profile alongside tadalafil and vardenafil provides valuable perspective. Although all share the PDE5 inhibition mechanism, subtle differences in selectivity, half-life, and tissue distribution yield distinct AE patterns.
- Tadalafil (longer half-life, greater PDE11 inhibition) tends to cause more myalgia and back pain.
- Vardenafil may provoke QT interval prolongation at high doses.
- Sildenafil shows a unique association with visual disturbances due to higher PDE6 cross-reactivity.
The FAERS analysis reaffirmed these distinctions, supporting the notion that not all PDE5 inhibitors are pharmacologically equivalent, even if they are therapeutically interchangeable.
Strengths and Limitations of Real-World Data
The strength of this pharmacovigilance study lies in its scale and granularity — millions of patient experiences distilled into a comprehensive risk panorama. However, interpretation must remain cautious.
Strengths
- Large dataset encompassing diverse demographics and comorbidities.
- Detection of rare and long-term AEs not captured in clinical trials.
- Dynamic surveillance reflecting evolving usage patterns (including off-label indications).
Limitations
- Underreporting bias: Only a fraction of adverse events are formally reported.
- Causality ambiguity: Association does not imply direct cause-effect linkage.
- Incomplete clinical context: Missing data on dosage, timing, and concomitant illnesses.
Despite these caveats, pharmacovigilance data remain an indispensable complement to randomized controlled trials, bridging the gap between efficacy under ideal conditions and safety under real-world complexity.
Clinical Implications: Managing Risk While Preserving Benefit
The study’s take-home message is not one of alarm but of clinical pragmatism. Sildenafil remains an extraordinarily effective and generally well-tolerated therapy — provided it is prescribed and monitored intelligently.
Practical Recommendations
- Assess cardiovascular risk before initiating therapy. Avoid in unstable angina or recent myocardial infarction.
- Review concomitant medications, especially nitrates, alpha-blockers, and potent CYP3A4 inhibitors.
- Educate patients about visual, auditory, or neurologic warning signs.
- Report adverse events promptly to national pharmacovigilance systems to strengthen collective safety data.
These steps transform pharmacovigilance from a passive data exercise into active clinical stewardship.
Beyond Erectile Dysfunction: The Expanding Sildenafil Landscape
The FAERS data also highlight the drug’s diversifying use profile. Beyond ED and PAH, sildenafil is increasingly explored in Raynaud’s phenomenon, altitude sickness, and female sexual arousal disorders. Each new indication introduces unique pharmacodynamic interactions and safety considerations.
The future of sildenafil may therefore lie not only in male reproductive medicine but in systemic vascular and metabolic modulation. The same NO–cGMP axis that governs penile erection also influences microvascular tone, mitochondrial efficiency, and cellular resilience — pathways increasingly relevant in cardiometabolic and neurodegenerative disease research.
Conclusion
Sildenafil’s post-marketing journey is a case study in pharmacological evolution. From an accidental discovery in the 1990s to a cornerstone of cardiovascular and sexual medicine, it continues to reveal new dimensions of efficacy and safety.
The real-world pharmacovigilance analysis from the FAERS database reinforces what decades of clinical practice have shown: sildenafil is generally safe but not innocuous. Its risks are predictable, manageable, and — when contextualized within pathophysiology — instructive.
Ultimately, the key to optimizing sildenafil therapy lies not in fear of adverse events but in understanding their mechanistic roots. By integrating pharmacovigilance insights with clinical judgment, healthcare professionals can preserve the benefits of this remarkable molecule while safeguarding patients from its pitfalls.
FAQ
1. What are the most common adverse events associated with sildenafil?
The most frequently reported events involve the cardiovascular, visual, and gastrointestinal systems — including headache, flushing, hypotension, visual color changes, and dyspepsia. These effects are typically dose-dependent and transient.
2. Are there serious or life-threatening risks?
Serious events such as myocardial infarction, sudden hearing loss, and NAION are rare but documented. They generally occur in patients with preexisting vascular disease or in cases of inappropriate co-administration with nitrates or CYP3A4 inhibitors.
3. How can clinicians use pharmacovigilance data in daily practice?
Pharmacovigilance insights help clinicians anticipate potential drug interactions, identify emerging risks, and personalize therapy. Regularly reviewing safety data and reporting new adverse events enhances the collective knowledge base and patient safety.